Document 13260969
advertisement

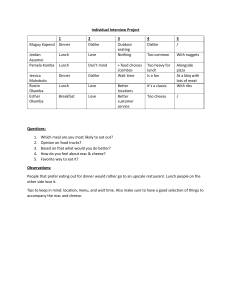
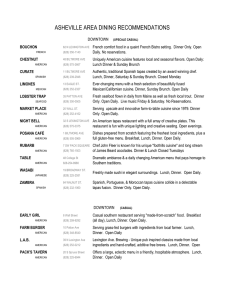
MCRTP 2016 Workshop Schedule1 Time 8:00 – 8:30 8:30 – 9:30 9:30 – 10:30 10:30 – 10:45 10:45 – 12:00 12:00 – 1:00 1:00 – 1:15 1:15 – 2:15 2:15 – 3:15 3:15 – 3:30 3:30 – 5:30 5:30 – 6:00 6:00 – 8:00 8:00 1 2 Day 1 – Wed, July 13 Open Day 2 – Thu, July 14 Open Day 3 – Fri, July 15 Open Day 4 – Sat, July 16 Open NIH supported clinical trials and networks Clinical trial designs (Phase 1, 2, and 3 studies) Systematic literature review Descriptive statistics and 2 statistical inference Patient reported outcomes & quality-­‐of-­‐life studies Survival analysis Break Break Break Break Defining the hypothesis Correlation & linear regression, 2 sample size and power Research grant writing Biostatistics practicum Intro project presentations Lunch Time management Break Lunch Health services research and health care policy Break Lunch Getting your clinical trial open Break Data management Logistic regression, bias and 2 confounding Case studies: learning from the mistakes of others Observational trial designs (cohort and case control studies) Demystifying the regulators Registry studies Break Break Break Small group session Small group session Small group session Break Break Dinner How to be successful in research Dinner Tricks of the Trade Close Close 2 2 Lunch Developing and managing industry relations Break Updated project presentations Close Close Lecture topics and times are subject to change. Laptop is required for use of Stata software package (software will be provided by MCRTP program).