Proposal Submissions (NIH, State, Foundations) Abbreviated Checklist SUGGESTED GUIDELINES
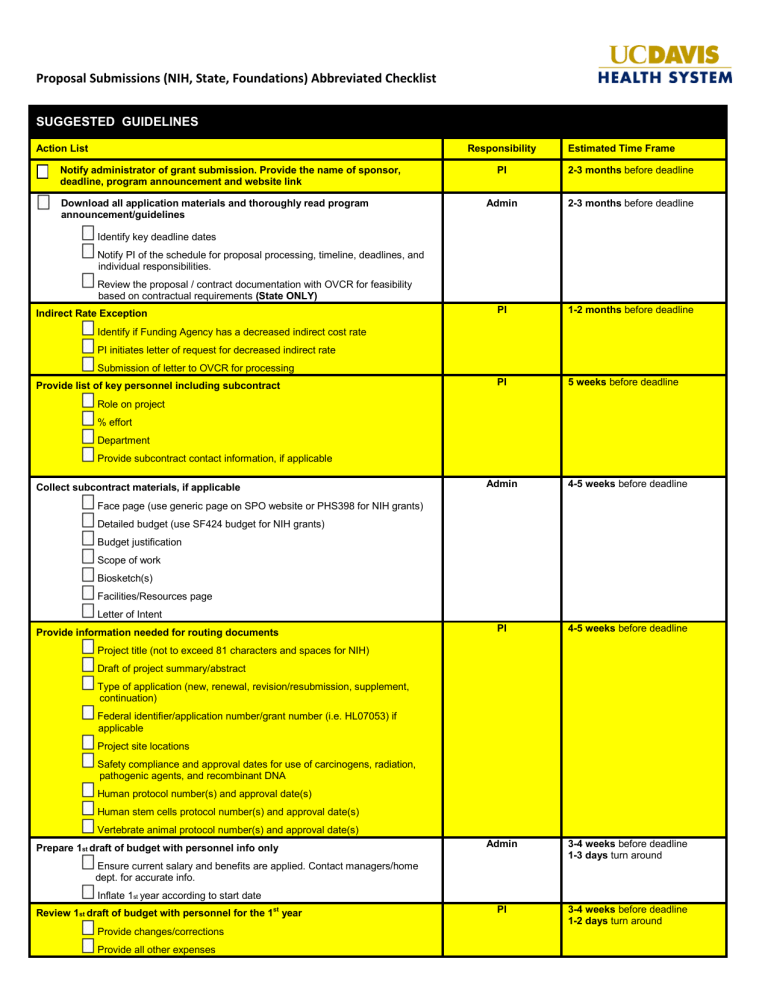
Proposal Submissions (NIH, State, Foundations) Abbreviated Checklist
SUGGESTED GUIDELINES
RESPONSIBILITIES
LEAD TIME
Action List
Notify administrator of grant submission. Provide the name of sponsor, deadline, program announcement and website link
Download all application materials and thoroughly read program announcement/guidelines
Identify key deadline dates
Notify PI of the schedule for proposal processing, timeline, deadlines, and
individual responsibilities.
Review the proposal / contract documentation with OVCR for feasibility
based on contractual requirements
Indirect Rate Exception
(State ONLY)
Identify if Funding Agency has a decreased indirect cost rate
PI initiates letter of request for decreased indirect rate
Submission of letter to OVCR for processing
Provide list of key personnel including subcontract
Role on project
% effort
Department
Provide subcontract contact information, if applicable
Collect subcontract materials, if applicable
Face page (use generic page on SPO website or PHS398 for NIH grants)
Detailed budget (use SF424 budget for NIH grants)
Budget justification
Scope of work
Biosketch(s)
Facilities/Resources page
Letter of Intent
Provide information needed for routing documents
Project title (not to exceed 81 characters and spaces for NIH)
Draft of project summary/abstract
Type of application (new, renewal, revision/resubmission, supplement, continuation)
Federal identifier/application number/grant number (i.e. HL07053) if
applicable
Project site locations
Safety compliance and approval dates for use of carcinogens, radiation, pathogenic agents, and recombinant DNA
Human protocol number(s) and approval date(s)
Human stem cells protocol number(s) and approval date(s)
Vertebrate animal protocol number(s) and approval date(s)
Prepare 1 st draft of budget with personnel info only
Ensure current salary and benefits are applied. Contact managers/home
dept. for accurate info.
Inflate 1 st year according to start date
Review 1 st draft of budget with personnel for the 1 st
year
Provide changes/corrections
Provide all other expenses
Responsibility
PI
Admin
PI
PI
Admin
PI
Admin
PI
Estimated Time Frame
2-3 months before deadline
2-3 months before deadline
1-2 months before deadline
5 weeks before deadline
4-5 weeks before deadline
4-5 weeks before deadline
3-4 weeks before deadline
1-3 days turn around
3-4 weeks before deadline
1-2 days turn around
Proposal Submissions (NIH, State, Foundations) Abbreviated Checklist
Prepare 2 nd draft of budget with all expenses for entire project period
Include inflation of 3% salary for each year
Include OVCR approved benefit rates for year (State ONLY)
Include negotiated composite benefit rates for specific project period and add 3% inflation for each year beyond currently published rates
(NIH/Foundation ONLY)
Include inflation of 10% for remission fees
Ensure accurate indirect cost rates are applied
Prepare cost sharing form, if needed, and obtain approval from department chair if required.
Review 2 nd draft of budget for entire project period
Provide changes
Provide all other expenses
Prepare final draft of budget for entire project period
Review final draft of budget for entire project period
Provide budget justification
Prepare and route signature pages
OVCR
SOM
Datasheet
Transmittal Form
Form 800 and/or 700U
Subcontract forms, if applicable
Resource Page (NIH/Foundation ONLY)
Budget and Budget Justification (NIH/Foundation ONLY)
Update biosketch and other/research support pages
Collect and format biosketches from all key personnel
Collect and format other/research support from all key personnel, if applicable
Assemble the grant application per sponsor’s guidelines as documents are received
Submit completed grant application and signed data sheets to SOM SPO for review
Confirm receipt of the proposal at OVCR and assigned to an analyst
Finalize the application file
Provide submission for review to PI—electronic or hard copy
Make corrections as required
Upon PI approval notify OVCR assigned analyst of submission file
readiness
Submit final submission file electronically to OVCR
OVCR to authorize and submit to the funder (in some cases the PI will
submit the final file via email or federal express to the funder)
Admin 3 weeks before deadline
1-3 days turn around
PI 3 weeks before deadline
1-2 days turn around
Admin
PI
PI
Admin
3 weeks before deadline
3 weeks before deadline
3 weeks before deadline
2-3 weeks before deadline
PI
Admin
Admin
Admin
Admin
Admin
Admin
2-3 weeks before deadline
2-3 weeks before deadline
2-3 weeks before deadline
2-3 weeks before deadline
7-10 days before deadline
3-5 days before deadline
3-5 days before deadline