Flexicates: Towards α-helix Functionality through Unsymmetrical Self-Assembly PhD began: October 2010
advertisement

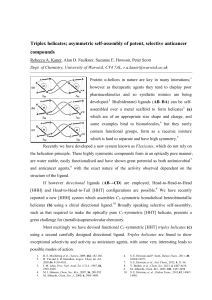
Flexicates: Towards α-helix Functionality through Unsymmetrical Self-Assembly Rebecca A. Kaner*, Dr. Suzanne E. Howson, Alan D. Faulkner and Prof. Peter Scott Department of Chemistry, University of Warwick, Coventry, UK, CV4 7AL PhD began: October 2010 We will present new strategies to generate optically pure compounds with unprecedented, α-helix-like complexity via iron-templated selfassembly. Protein -helices I are employed in binding events with proteins, nucleic acids and membranes. They are a major component of most host-defence peptides2 and mediate key protein handshakes in cancer.3 As therapeutic agents, however, they tend to rapidly degrade leading to poor pharmacokinetics and so synthetic mimics are being developed which include more favourable properties.4 The metal-templated triple bimetallic helicates II are of a similar size and charge to -helices and some bind to biomolecules,5 but they rarely contain functional groups and have much higher symmetry (Cn). Overall, the chemistry is currently not sophisticated enough to deliver molecules that fulfil the criteria which would allow them to be considered as practical -helix mimics in biomedical chemistry.6 I II III (a) Antibacterial α-helix of human cathelicidin LL-37 of similar size and charge density to a flexicate;1 (b) traditional 3-fold symmetric helicate [M2(AB'-B'A)3]; (c) mixed-ligand, low symmetry, functionalized flexicate. We recently developed a series of optically and diastereochemically pure monometallic complexes [M(AB)3]2+ 7,8 and this project tethers these with various linkers to create water stable, optically pure structures [M2(AB'-B'A)3]4 which we call flexicates by analogy with the related helicates. They have potential in the biological regime as antimicrobial9 and anticancer agents, with selectivity and activity determined by the exact functionality of the organic ligand. Initial IC50 values have shown that flexicates have a similar activity to Cisplatin with a significantly better selectivity profile and we will report many new functional complexes. Current synthetic approaches to self-assembly invariably lead to either symmetrical complexes (as II) or more commonly mixtures. Most excitingly we have devised functional L1 X: CH2OH, L2 X: CH2Naph unsymmetrical flexicates III using carefully designed directional ligands ABCD. This new approach has allowed us to take hitherto unfeasible steps towards -helix-like complexity in a readily-assembled large molecule, and thus make major progress in the development of very novel drug candidates. L3 (AB'-B'A) L4 (AB-CD) DFT calculated structure of [M2(AB-CD)2(CD-BA)]4+ and MD calculated structure of [M2(AB'-B'A)3]4+ 1. 2. 3. 4. X. Li, et al., J. Am. Chem. Soc., 2006, 128, 5776-5785. R. E. W. Hancock and H.-G. Sahl, Nat. Biotech., 2006, 24, 1551-1557. R. E. Moellering et al., Nature, 2009, 462, 182-188. H. Yin and A. D. Hamilton, Angew. Chem. Int. Ed., 2005, 44, 4130-4163. 5. 6. 7. 8. 9. M. J. Hannon, Chem. Soc. Rev., 2007, 36, 280-295. S. E. Howson and P. Scott, Dalton Trans., 2011, 40, 1026810277. S. E. Howson, et al., Chem. Commun., 2009, 1727-1729. S. E. Howson, et al., Dalton Trans., 2011, 40, 10416-10433. S. E. Howson, et al., Nat. Chem., 2012, 4, 31-36.