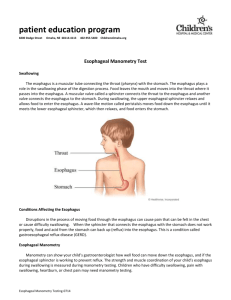
AN ABSTRACT OF THE THESIS OF
advertisement

AN ABSTRACT OF THE THESIS OF Peerapol Sukon for the degree of Doctor of Philosophy in Comparative Veterinary Medicine presented on April 19, 2002. Title: The Physiology and Anatomy of the Esophagus of Normal Llamas and Llamas with Megaesophagus. Abstract approved: Signature redacted for privacy. Karen I. Timm Solid-state esophageal manometry was used to study physiology of the normal llama esophagus. Esophageal baseline pressures, esophageal response to water swallows and to balloon distention, and other motor activity of the esophagus during experiments were measured throughout the entire length of the esophagus. Gross and histologic anatomy, muscle fiber type classification, and ultrastructural morphology were used to study anatomy of the normal llama esophagus. The methods as described were also applied to study the physiology and anatomy of two llamas with megaesophagus and one llama with esophageal motor abnormalities. Primary peristalsis in response to a water swallow is unique in the llama esophagus. Duration of contraction is very short. Propagation velocity of the peristaltic wave is fast, approximately ten times of that of the human. Amplitude of contraction and mean rate of pressure change per unit time are the greatest in the pharyngoesophageal region and the lowest in the lower esophageal sphincter region. Length of the cervical portion of the esophagus is almost twice that of the thoracic portion in keeping with long neck. Submucosal glands are abundant throughout the esophagus. Type 2 muscle fibers are predominant throughout the esophagus. Type 1 muscle fibers are also present and gradually increase in the distal portion of the esophagus. Generally, ultrastructure of the striated muscle of the esophagus is similar to that of the skeletal muscle. Primary peristalsis was abnormal in llamas with megaesophagus. More than a half of the distal esophagus was aperistaltic. Manometric findings corresponded to anatomical findings. Esophageal dilation was remarkable in the distal portion of the esophagus. Evidence of denervation atrophy of the esophageal striated muscle was not found in the cranial cervical region, but was found in the middle cervical region through the distal thoracic region. This study provides extensive normal data on the llama esophagus to serve as a baseline for further study of esophageal pathology in the llama. Findings from this study suggest that denervating disease is a cause of megaesophagus in the llama, although the exact nature of this disease is still unknown. ©Copyright by Peerapol Sukon April 19, 2002 All Rights Reserved The Physiology and Anatomy of the Esophagus of Norma' Llamas and Llamas with Megaesophagus by Peerapol Sukon A THESIS Submitted to Oregon State University In partial fulfillment of the requirements for the degree of Doctor of Philosophy Presented April 19, 2002 Commencement June 2002 ACKNOWLEDGMENTS First, I would like to thank my major professor, Dr. Karen I. Timm, for guiding and supporting me in my research efforts during my graduate career. I also would like to acknowledge the remaining members of my graduate committee: Dr. Beth A. Valentine, Dr. Bradford B. Smith, Dr. Jerry R. Heidel, and Dr. Robert T. Mason. I am grateful for their contributions to the direction and content of my research efforts. I would like to thank Kay Fischer for helping with electron microscopic technique. Dr. Barry J. Cooper examined and interpreted the vagus nerve sections. I would like to thank the Royal Thai Government, the Willamette Valley Llama Foundation, the Department of Biomedical Sciences, and Oregon State University for financial support. Finally, I would like to thank my family for their unconditional support. CONTRIBUTION OF AUTHORS Dr. Karen I. Timm was involved in the experimental design, analysis, and writing of each chapter. Dr. Beth A. Valentine provided muscle histochemical technique used in Chapter 3 and 4, assisted in data analysis, and prepared manuscript for publication. Dr. Bradford B. Smith helped with experimental design and data analysis in Chapter 2. TABLE OF CONTENTS Page CHAPTER 1. GENERAL INTRODUCTION . TOPICS AND PROBLEMS GOALS AND OBJECTIVES 2 RESEARCH APPROACH 3 PREVIOUS STUDIES RELATED TO THE THESIS 8 REFERENCES 14 CHAPTER 2. ESOPHAGEAL PHYSIOLOGY OF LLAMAS (Laina giarna) 20 ABSTRACT 20 INTRODUCTION 21 MATERIALS AND METHODS 23 RESULTS 31 DISCUSSION 43 REFERENCES 53 CHAPTER 3. A GROSS, HISTOLOGIC, MUSCLE HISTOCHEMICAL, ULTRASTUCTURAL STUDY OF THE ANATOMY OF THE LLAMA ESOPHAGUS .57 ABSTRACT INTRODUCTION 57 .. .58 MATERIALS AND METHODS 59 RESULTS 64 TABLE OF CONTENTS (Continued) Page DISCUSSION REFERENCES CHAPTER 4. CASE REPORT: TWO LLAMAS WITH MEGAESOPHAGUS AND ONE LLAMA WITH ESOPHAGEAL MOTOR ABNORMALITY 71 .. .79 .82 ABSTRACT 82 INTRODUCTION 83 CASE REPORTS 84 RESULTS 87 DISCUSSION 100 REFERENCES 104 CHAPTER 5. GENERAL CONCLUSION 108 BIBLIOGRAPHY 111 LIST OF FIGURES Figure 1.1 2.1 2.2 Page The failure of the water-filled catheter system for esophageal manometry in the llama 5 A representative tracing demonstrating calculation of peristaltic parameters . Box and whisker plot of the amplitude of contraction (mmHg) of the esophagus during water swallows of ten llamas .30 33 2.3 Box and whisker plot of the duration of contraction (s) of the esophagus during water swallows of ten llamas 34 2.4 Box and whisker plot of the mean rate of pressure change per unit time (nmiHgls) of the esophagus during water swallows of ten llamas .35 Box and whisker plot of the propagation velocity (cm/sec) of the esophageal peristaltic wave during water swallows of ten llamas 36 2.5 2.6 Cl contraction generating a burp into the esophagus 2.7 Secondary peristalsis occurring immediately after a burp in the thoracic portion of the esophagus 41 2.8 Deglutitive inhibition 3.1 A typical cross section of the llama esophagus 3.2 Myosin ATPase stained sections of the llama esophagus and limb (vastus intermedius) muscle (lOOx) 3.3 Esophageal striated muscle by transmission electron microscopy 4.1 Resting pressures in the cervical (A) and thoracic (B) esophagus of case 1 ..40 .42 . .67 70 ..71 88 LIST OF FIGURES (Continued) Figure 4.2 Page Station pull-through technique shown the abnormal resting pressure of the esophageal body in case 3 89 4.3 Failure of a swallow to initiate esophageal peristalsis 91 4.4 Primary esophageal peristalsis in a llama with megaesophagus 92 4.5 Plots of means of the amplitude of contraction from a normal llama and all three cases 94 4.6 Incomplete peristalsis in case 3 95 4.7 Myosin ATPase stained sections at pH 10 of esophageal striated muscle and limb (vastus intermedius) muscle from the normal llamas (left side) and llamas with megaesophagus (right side) (lOOx) 99 LIST OF TABLES Table Page 2.1 Results of manometnc evaluation in ten normal llamas 37 2.2 Response of the llama esophagus to balloon distention 39 3.1 Summary of the length and outer diameter of the llama esophagus from 25 llamas 65 The thickness of tunica muscularis and the number of lobules of submucosal glands in each region of the esophagus 68 Diameter of the esophagus and thickness of the tunica muscularis in various regions throughout the length .96 3.2 4.1 THE PHYSIOLOGY AND ANATOMY OF THE ESOPHAGUS OF NORMAL LLAMAS AND LLAMAS WITH MEGAESOPHAGUS CHAPTER1. GENERAL INTRODUCTION TOPICS AND PROBLEMS The llama (Lama glama), a South American Camelid (SAC) belonging to the family Camelidae and suborder Tylopoda, is native to the Andean highlands. The mature llama has an average body weight of 155 kilograms and historically was used as a pack animal. Importation of llamas to North American began in the early 1900s and the estimated number in 1997 was 120,000 - 140,000 (Smith, 1998). The most common esophageal problem in llamas is megaesophagus, a term referring to generalized esophageal dilation. Overall incidence is difficult to determine. In an informal survey of veterinarians in the Northwest who routinely treat SAC's it was found that most were aware of at least one SAC with megaesophagus in their practice area. In a retrospective study of 15 llamas with acquired megaesophagus animals ranged in age of onset from 13 months to 9.5 years. Etiology was not determined in 11 animals. Megaesophagus in one llama was thought to be due to organophosphate toxicity, vagal neuropathy was suspected in one, and degenerative myopathy was found in two llamas (Watrous et al., 1995). Megaesophagus in a nine year old, male llama was reported with persistent right aortic arch (Butt et al., 2001). There are no reports in the literature of anatomic and 2 physiologic studies of the llama esophagus. There is one anatomic study of the submucosal glands and musculature of the camel esophagus (Jamdar and Ema, 1982). As little is known about the normal esophagus in a llama, to study megaesophagus it is first necessary to thoroughly study the normal llama esophagus. The many questions to answer are: What is the orientation of muscle fibers in the tunica muscularis and is it similar to that of other species or to that of other gastrointestinal regions? Are there any nerve plexus and ganglion cells present in the tunica muscularis? What are muscle fiber types of the esophageal striated muscle? How do the muscle fiber types distribute throughout the esophagus? What is the distribution of submucosal glands throughout the esophageal length compared to other species? Are the upper and lower esophageal sphincters present in llamas? If present, are they different from other species? How does the esophagus respond physiologically to water swallows? What are the characters of primary peristalsis throughout the esophagus? How fast is the propagation velocity of the peristaltic wave? How short is the duration of contraction during peristalsis? What is the nature of secondary peristalsis? GOALS AND OBJECTIVES This study focuses on the physiology and anatomy of the normal llama esophagus providing a thorough description. The normal is then used as a baseline for study of megaesophagus cases. The objectives of this study are: (1) describe the physiology of normal llama esophagus, (2) describe the anatomy, including gross, 3 histologic, muscle histochemical, and ultrastructural features of the normal esophagus, and (3) study megaesophagus and esophageal motor activity problems in comparison to the normals with the hope of determining an etiology of megaesophagus in the llama. RESEARCH APPROACH Procedure Ten normal llamas, two llamas with long duration megaesophagus and one llama with esophageal motility abnormalities were studied. Megaesophagus was diagnosed based on clinical signs, and was confirmed with radiographs. This study was conducted in two main steps, a study of the normal llama esophagus and then of the megaesophagus animals. The esophageal anatomy and physiology of the two groups of animals were compared. Methods Objective (1) The physiology of normal llama esophagus Esophageal manometry was used to study the physiology of the normal esophagus. This technique can assess the motor activity of the esophagus both during swallowing and resting states. By measuring esophageal pressure complexes, it has proven to be a useful tool in both clinical and basic research 4 laboratories in the human and opossum (Castell, 1995). In preliminary work with the llama, an intraluminal esophageal manometric system with a water-filled catheter was used. The catheter was designed and assembled with 4 recording channels 10 cm apart. The appropriate flow rate of water through the catheter was determined for the highest accuracy. Unfortunately, this system did not work in llamas. During esophageal peristalsis, there was too much compliance in the system and too slow a response time of the system for adequate pressure recordings (Figure 1.1). Another problem was the difficulty of measuring the baseline pressure in the cervical segment of the llama esophagus due to the long neck and the effect of gravity on the water in the system. Llamas will not voluntarily lie in a recumbent position to remove this gravity effect. 5 jnzL 14L-..L iTRi Utfii" W'! hfliij' juir t ni'imijiii iWPllil? '!PllV4'lllltlH iwu nsrin ai uiii ;r U!d'lHLd llJ!Oil1llfflHHhJIllffiIUflflHJRR lltQijUIfltuuFllflIPJI1 Ill II 'MrS1iuIa1iiu 1"lv"qH!i!u'wnJIqv otiiSriiiinu II U WPJRUIthP 'iffill !llllapQrt qj jjji ip11aa tIIbiILII!fflPIIIuII1IEIIIIW1i iu1 'II 4 lii V IIIIIRIIH1IIIIII8IIilJIIKPIII1!'HIJIMPIIIHHIHIIIHIIHIIffiIIMØff1HWHUIMNiThUJ ii?ig lIlHh1UdflRllYlHWllhYBKIllllllffluIffffWWulM .tllftwwqø '79fF mlluubuIftrnufflifflI Li9PllfflMiWllllfflIdIllhhIftlUO III !IIIIII1 i!iidiirnrwimmiisuornuiuui;rnmnn;immwn H IIJI1W1RIIH 1ilL 'I11"#thffl jAIM1ullr. £ uqgi ullifi!! IUThUCIIj fiiiiiluIUH Ull hit flflci!uigqrijjj q s4ff!llllft iiffIUllMhiiiIIffu1hr I filM IfflbøII IIWfHIIMd1J fl i I!'li!t4c' iJCt :a W'f 1 rTj lliffDffjEUflh1MItNilhu,, bfl1 'ap'j;':;&Jllil r.'--- I iri--, I!9AaIIWIWIt1iI1ill IMILII illiIiFUllffiR1IW hi mfç2hr !"ju :i Jm ii' in .ar5,ru.n raiiw*wnoinin 194 ijj[jm:ffffihJ ffIIIIIIJI,J ffuillUllU flflfflI9flifflBfldJCflilDl t' 11IIIIÜ1MIIUW UilIilhPIPII IIUW4IIUW11 Ji M ffidM .ilE1J HhHFfl;flO'JffidHpwW1 III1U llfflffImJffil i IP1III t I Eli fl ajWq wukffilffl I imp i 11111 q ijnhi cuhttil , -. i,updL,aptrn guiLuulu idh Figure 1.1 The failure of the water-filled catheter system for esophageal manometry in the llama. When the contractions of the esophagus were strong such as in the upper and lower tracings, the system failed (arrow heads) to record the proper amplitude of contractions. Note the notch rather than full amplitude of contraction. Although this system works in humans, it does not work in llamas because the tunica muscularis in llamas consists of striated muscle. Unlike the smooth muscle in humans, the contraction of striated muscle in the llama esophagus is much faster, requiring a high response rate of the manometric system. Thus, a solid-state esophageal manometry catheter in which the microtransducers are contained and 6 directly measure the esophageal contractions was used for the entire study (Model CT/S4L, Medical Measurements Inc., Hackensack, NJ). The system was tested in a horse and a cow to compare the results with previous work (Clark et al., 1987) and validate the system. The results of the test were similar to previous studies only in the distal region of the horse esophagus, which consisted of smooth muscle. Results differed from the previous studies in the cow esophagus and upper region of the horse esophagus, which consisted of striated muscles. The duration of contraction and peristaltic velocity were shorter and faster respectively than that reported in the previous studies in the horse and cow (Clark et al., 1987). The previous studies in the cow and horse used a water-filled catheter system. It has been stated that different manometry systems will give quanitatively different results (Herwitz et al., 1979). Thus, clinically normal baselines for each manometry system must be established. Manometry was performed on standing awake animals. The resting pressure of the upper esophageal sphincter, esophageal body, and lower esophageal sphincter was recorded. The peristaltic velocity, the amplitude of contractions, and the duration of contractions were recorded after water swallows. Secondary peristalsis was studied by a technique of intraluminal balloon distention. Objective (2) The anatomy of normal llama esophagus Task 1. Gross anatomy. After being studied by esophageal manometry, the llama was humanely killed. The diameter of esophagus was measured at cranial cervical, middle cervical, thoracic inlet, middle thoracic, and distal thoracic 7 regions. The entire length and length of the cervical and thoracic portions of the esophagus were measured. Task 2. Microscopic anatomy. The cranial cervical, middle cervical, thoracic inlet, middle thoracic, and distal thoracic regions of the esophagus were fixed in 10% formalin for routine histological sections. The sections were examined with a light microscope to determine the general structure of the esophagus. Orientation of muscle fibers and number of submucosal glands were described. Task 3. Muscle Histochemistry. The esophageal striated muscles were collected from each region as defined above. The esophageal striated muscle fiber types of frozen sections were determined by histochemical staining for myosin ATPase activity. Task 4. Ultrastructure. The ultrastructure of esophageal striated muscle and associated nerves in normal llamas was investigated by transmission electron microscopy. Objective (3) Compare two megaesophagus and one esophageal motor problem cases Megaesophagus and esophageal motor activity problem cases were studied in the same manner as the normal llamas and compared to the normal llamas. 8 PREVIOUS STUDIES RELATED TO THE THESIS The esophagus is a tubular organ of the gastrointestinal tract. The primary function of the esophagus is to propel the food or fluid boluses from the pharynx into the stomach (first compartment in the llama) and in llamas, as in the ruminants, to carry the regurgitated bolus back to the mouth for rechewing and swallowing. Like other regions of alimentary tract, the esophageal wall consists of mucosa, submucosa, muscular layers. However, unlike the other regions it has no true serosal layer. The outer coat is adventitia. Only small areas (lateral sides) of the thoracic segment and the very short abdominal segment are covered with a serosal coat. The mucosa consists of stratified squamous epithelium. The degree of keratinization of stratified squamous epithelium varies with the species and relates to the character of food: coarse food, highly keratinized; soft food, slightly keratinized to nonkeratinized. The epithelium is usually nonkeratinized in humans, other primates, and carnivores, slightly keratinized in the pig, more in horse, and keratinized to a high degree in ruminants (Stinton and Calhoun, 1993). The tunica submucosa consists of loose connective tissue containing blood vessels, nerve fibers, lymphatics, and submucosal glands. It is clear that there are marked species differences with respect to the submucosal glands. For example, they are present within the esophageal wall of the human, opossum, dog, raccoon, and guinea pig, but are not found in the esophageal wall of the horse, cat, rat, and rabbit (Goetsch, 1910). In the ruminants (cattle, sheep, and goat), the submucosal g'ands are present in the pharyngoesophageal region only (Dellmann, 1971; Stinton 9 and Calhoun, 1976). In contrast, the glands are abundant and found throughout the length of the camel esophagus (Jamdar and Ema, 1982). The tunica muscularis varies greatly from species to species. In humans, pigs, horses, opossums, and cats the muscularis of the upper or cranial part of esophagus consists of striated muscle but the lower or caudal region consists of smooth muscle (Goetsch, 1910; Schummer et al., 1979). In mice, rats, rabbits, dogs, and ruminants the tunica muscularis of the esophagus consists almost entirely of striated muscle (Goetsch, 1910; Schummer et al., 1979). With the difference in muscle types of the tunica muscularis, there is a difference in innervation and esophageal physiology. For example, the speed of esophageal penstalsis is much faster in dogs (9 to 10 cmlsec) (Satchell, 1990) than in cats (1 to 2 cm/see) (Washabau, 1992). Autonomous peristalsis, peristalsis in the absence of extrinsic innervation, can occur in the smooth muscle portion but not in the striated muscle portion of the esophagus (Burgess et al., 1972; Greenwood et al., 1962). The esophagus and its sphincters are controlled by efferent nerve fibers that arise from cell bodies located in the motor vagal nuclei of the brain stem. The somatic motor fibers to the striated muscle arise from neurons located in the nucleus retrofacialis and most rostral portion of the nucleus ambiguus (Fryscak et al., 1984; Hoistege et al., 1983; Lawn, 1964; Yoshida et al., 1981; Hudson and Cummings, 1985). The preganglionic fibers that innervate the smooth muscle portion of the esophagus and lower esophageal sphincter arise from cell bodies located in the dorsal motor nucleus of the vagus as well as from cells located in the nucleus ambiguus (Barone et al., 10 1984; Fryscak et al., 1984). Although the esophagus may be considered a relatively simple organ in anatomy and function, the control mechanisms are far from simple (Diamant, 1997). The esophagus has been well investigated in humans, rats, mice, opossums, dogs, and cats. Recent research has been focused on the development and innervation of esophageal striated muscle. During development, the external muscle of the mouse esophagus undergoes a transdifferentiation from smooth to striated muscle (Patapoutian et al., 1995). Cells exhibiting striations were first observed in the outer layers of the most rostral regions of the esophagus of embryonic day 15 mice. Clusters of nicotinic acetyicholine receptors were first observed at the rostral end of the esophagus of embryonic day 15 mice, and developed in rostrocaudal progression that coincided with the appearance of striations within the myofibers (Sang and Young, 1997). There is evidence for coinnervation between extrinsic vagal terminals and intramural (enteric) ganglia on motor endplates of rat and guinea-pig esophageal striated muscles (Kuramoto et al., 1996; Zhou et al., 1996; Won et al., 1997; Won et al., 1998; Morikawa and Komuro, 1998; Neuhuber et al., 2001). Fiber typing of esophageal striated muscle by histochemistry has been done in guinea-pig, marmoset, macaque, and human (Whitmore, 1982). The guinea-pig esophageal striated muscle was found to be of one type, "fast twitch" oxidative and glycolytic (type hA). Both macaque and human each had two types: "slow twitch" oxidative glycolytic (type I) and "fast twitch" oxidative glycolytic (type hA), and "slow twitch" oxidative (type I) and "fast twitch" putatively glycolytic (type JIB), 11 respectively. It was concluded that these differences represent species variation. Histochemistry of esophageal striated muscle in opossums and humans was investigated (Shedlofsky-Deschamps et al., 1982). In both species, the striated muscle was type II fibers throughout, but at the pharyngeal end, some type I fibers passed into the tunica muscularis from the caudal pharyngeal constrictor and extended for a short distance along the esophagus. Differences in the reactions to the histochemical stains indicated that the type II fibers of the opossum esophageal striated muscle were subtype A (type hA), while those of the human were subtype B (type JIB). The fiber types of the striated muscle of esophagus of the cow, sheep, donkey, dog, and cat were examined (Mascarello et al., 1984). In the ruminants and donkey the muscularis was composed of fiber types I, hA, and IIC. In the ruminants there was a gradient in the proportion of type I fibers from 1% (at the cervical end) to about 30% (at the caudal end). Histochemistry on the cervical, thoracic, and abdominal esophageal muscle of immature, young, and adult normal dogs revealed type hA fibers (Hudson, 1993). Ultrastructure of the esophagus has been investigated in many species. The ultrastructure of the canine distal esophagus was studied focusing on the interstitial cells of Cajal and their relationship to nerves and muscles (Berezin et al., 1994). The quantitative ultrastructural anatomy of the esophagus in different regions in the horse was studied to determine the effect of specimen preparation (Slocombe, 1982). The ultrastructure of esophageal striated muscle fibers has been investigated in the human (Faussone-Pellegrini and Cortesini, 1987) and guinea-pig 12 (Whitemore, 1983; Whitemore and Notman, 1987). Ultrastructure of neuromuscular junctions of longitudinal and circular muscle fibers of the guineapig esophagus and their relation to the myenteric plexus has been studied (Zhou et a!, 1996; Morikawa and Komuro, 1999). Intraluminal manometry has been used for the evaluation of esophageal peristalsis and sphincter function. Accurate recording of intraluminal esophageal pressure is important for satisfactorily examining esophageal motor function. The catheter diameter, catheter length, and water infusion rate are examples of certain variables that affect recording fidelity. The major cause of recording error is "failure" of fluid delivery by the infusion pump during a pressure transient (Stef et al., 1976). A solid-state catheter has the advantage that, unlike the water infusion system, pressures are measured directly and are unrelated to the relative position of the measured animal and the equipment; therefore, studies may be performed with the animal in any position. In addition, the response time of the solid-state catheter is much faster than that of the water infusion system, making possible more accurate measurements of the esophageal striated muscle (Kahnlas et al., 1994; Castell, 1999). Previous studies in large domestic species include horses, cows, and sheep. Equine esophageal pressure profile was determined using a 4 side-hole water infusion catheter assembly. Four functionally distinct regions of the esophagus were demonstrated: cranial esophageal sphincter, "fast" and "slow" regions in the body of the esophagus, and lower esophageal sphincter (Stick et aL, 1983). Esophageal pressure events during deglutition were evaluated in healthy 13 adult horses, cows, and sheep by using a 3 side-hole water infusion catheter assembly (Clark et al., 1987). Megaesophagus is a term referring to generalized esophageal dilation. Megaesophagus in llamas was reported with an acquired form (age of onset ranged from 13 months to 9.5 years). Most cases did not have a definitive etiological diagnosis (Watrous et al., 1995). Megaesophagus has been reported more frequently in dogs than other species (Shelton et al., 1997). There are three distinct clinical entities associated with canine megaesophagus; congenital megaesophagus, acquired idiopathic megaesophagus, and secondary megaesophagus. In the study of 74 cases of megaesophagus in dogs due to hypomotility, 9 % were congenital, 74% idiopathic, and 17% secondary to a specific neuromuscular dysfunction (Twedt, 1995). Peripheral neuropathies, laryngeal paralysis, acquired myasthenia gravis, esophagitis, chronic or recurrent gastric dilation, and old age were associated with an increased risk of developing megaesophagus in dogs (Gaynor et al., 1997). Myasthenia gravis (MG) has been recently recognized to be the most common cause of secondary megaesophagus in dogs (Yam et al., 1996). Megaesophagus has been reported in the other species such as horses (Bowman et al., 1978; Murray Ct al., 1988;), cows (Anderson et al., 1984; Vestweber et al., 1985; Ross et al., 1986; Bargai et al., 1991), cats (Hoenig et al., 1990, Maddison and Allan, 1990), domestic ferret (Harms and Andrews, 1993), mice (Randelia and Lalitha, 1988), and a goat (Parish et al., 1996). A defect in any part of the neuromuscular pathway that controls swallowing can result in esophageal motor dysfunction. This pathway 14 consists of sensory receptors in the esophagus, afferent nerve fibers in the vagus and its branches, the brain stem, efferent nerve fibers in the vagus, the neuromuscular junction, and the esophageal striated musculature itself. REFERENCES Anderson NV, Vestweber JG. Hiatal hernia and segmental megaesophagus in a cow. JAmVetMedAssoc 1984;184:193-195. Bargai U, Nanthan AT, Pearl S. Acquired megaesophagus in a heifer. Vet Radiol 1991;32:259-260. Barone FC, Lombardi DM, Ormsbee III HS. Effects of hindbrain stimulation on the lower esophageal sphincter pressure in the cat. Am J Physiol 1984;247:G70-G78. Berezin I, Daniel EE, Huizinga JD. Ultrastructure of interstitial cells of Cajal in the canine distal esophagus. Can J Physiol Phamtacol 1994;72: 1049-1059. Bowman KF, Vaughan JT, Quick CB, et al. Megaesophagus in a colt. J Am Vet Med Assoc 1978;172:334-337. Burgess JN, Schiegel JF, Ellis FH. Effect of denervation on feline esophageal function and morphology. J Surg Res 1972;12:24. Butt TD, Macdonald DG, Crawford WH. Persistent right aortic arch in a mature llama. Vet Rec 2001;148:1 18-119. Castell JA. Esophageal manometry. In: Castell DO, Richter JE, eds. The Esophagus, 2"' ed. Philadelphia: Lippincott-Reven, 1995;1 13-152. Castell JA, Castell DO. Stationary esophageal manometry. In: Scarpignato C, Galmiche JP,eds. Functional Investigation in Esophageal Disease 1994: 109-129. Clark ES, Morris DD, Whitlock RH. Esophageal manometry in horses, cows, and sheep during deglutition. Am J Vet Res 1987;48:547-551. Dellmann HD. Digestive system. In: Veterinary Histology. Philadelphia: Lea and Febiger, 1971;153-154. 15 Diamant NE. Neuromuscular mechanisms of primary peristalsis. Am J Med 1997; 103:40s-43s. Faussone-Pellegrini MS, Cortesini C. Ultrastructure of striated muscle fibers in the middle third of the human esophagus. Histol Histopathol 1986; 1: 119-128. Fryscak T, Zenker W, Kantner D. Afferent and efferent innervation of the rat esophagus: a tracing study with horseradish peroxidase and nuclear yellow. Anat Embryol 1984;170:63-70. Gaynor AR, Shofer FS, Washabau RJ. Risk factors for acquired megaesophagus in dogs. JAm Vet Med Assoc 1997;211:1406-1412. Goetsch E. The structure of the mammalian esophagus. Am J Anat 1910;10:1-40. Greenwood RK, Schlegel JF, Code CF Ct al. The effect of sympathectomy, vagotomy and esophageal interruption on the canine gastroesophageal sphincter. Thorax 1962;17:310. Harms CA, Andrews GA. Megaesophagus in domestic ferret. Lab Anim Sci 1993;43:506-508. Herwitz AL, Duranceau A, Haddad JK. Esophageal manometric techniques. In: Smith LH, ed. Major Problems in Internal Medicine. Philadelphia: WB Saunders Co, 1979;27-33. Hoenig M, Mahaffey MB, Parnel GF, et al. Megaesophagus in two cats. J Am Vet Med Assoc 1990;196:763-765. Hoistege G, Graveland G, Bijker-Biemond C, et al. Location of motoneurons innervating soft palate, pharynx, and upper esophagus. Anatomical evidence for a possible swallowing center in the pontine reticular formation. Brain Behave Evol 1983;23:47-62. Hudson LC. Histochemical identification of the striated muscle of the canine esophagus. Anat Histol Embryol 1993 ;22: 101-104. Hudson LC, Cummings JF. The origins of innervation of the esophagus of the dog. Brain Res 1985;326:125-136. Jamdar MN, Ema AN. The submucosal glands and the orientation of the musculature in the oesophagus of the camel. J Anat 1982;135:165-171. 16 Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology 1994;107: 1865-1884. Kuramoto H, Kato Y, Sakamoto H, et al. Galanin-containing nerve terminals that are involved in a dual innervation of the striated muscles of the rat esophagus. Brain Res 1996;734:186-192. Lawn AM. The localization, by means of electrical stimulation, of the origin and path in the medulla oblongata of the motor nerve fibers of the rabbit esophagus. J Physiol 1964; 174:232-244. Maddison JE, Allan OS. Megaesophagus attributable to lead toxicosis in a cat. J Am Vet Med Assoc 1990;197:1357-1358. Mascarello F, Rowlerson A, Scapolo PA. The fibre type composition of the striated muscle of the esophagus in ruminants and carnivores. Histochemistry 1984;80: 277-288. Morikawa 5, Komuro T. Distribution of myenteric NO neurons along the guineapig esophagus. J Auton Nerv Syst 1998;74:91-99. Morikawa 5, Komuro T. Ultrastructure of intramural ganglia in the striated muscle portions of the guinea pig esophagus. J Anat 1999;195: 111-120. Murray MJ, Ball MM, Parker GA. Megaesophagus and aspiration pneumonia secondary to gastric ulceration in a foal. J Am Vet Med Assoc 1988;192:381-383. Neuhuber WL, Eichhorn U, Won J. Entenc co-innervation of striated muscle fibers in the esophagus: just a "hangover"? Anat Rec 2001;262(1):41-46. Parish SM, Middleton JR. Baldwin TJ. Clinical megaesophagus in a goat with thymoma. Vet Rec 1996;139:94. Patapoutian A, Wold BJ, Wagner RA. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science 1995;270: 1818-1820. Pflugfelder CM, Cardinet GH, Lutz H, et al. Acquired canine myasthenia gravis: immunocytochemical localization of immunes complexes at neuromuscular junctions. Muscle Nerve 1981; 4: 289-295. 17 Randelia HP, Lalitha VS. Megaesophagus in ICRC mice. Lab Anim 1988;22:23-26. Ross CE, Rebhun WC. Megaesophagus in a cow. J Am Vet Med Assoc 1986;188:623-624. Sang Q, Young HM. Development of nicotinic receptor clusters and innervation accompanying the change in muscle phenotype in the mouse esophagus. J Comp Neurol 1997; 386:119-136. Satchell PM. The neuropathic oesophagus. A radiographic and manometric study on the evolution of megaesophagus in dogs with developing axonal neuropathy. Res Vet Sci 1990;48:249-255. Schummer A, Nickel R, Sack WO. The alimentary canal. In: The Viscera of Domestic Mammals, New York: Springer-Verlag, 1979;99-202. Shedlofsky-Deschamps G, Krause WJ, Cutts JH et al. Histochemistry of the striated musculature in the opossum and human oesophagus. J Anat 1982;134:407414. Shelton GD, Schule A, Kass PH. Risk factors for acquired myasthenia gravis in dogs: 1,154 cases (1991-1995). JAm Vet Med Assoc 1997;211:1428-1431. Shelton GD, Willard MD, Cardinet GH, et al. Acquired myasthenia gravis: selective involvement of esophageal, pharyngeal, and facial muscle. J Vet Intern Med 1990; 4: 28 1-284. Slocombe RF, Todhunter RJ, Stick JA. Quantitative ultrastructural anatomy of esophagus in different regions in the horse; effects of alternate methods of tissue processing. Am J Vet Res 1982;43:1137-1142. Smith BB. Natural history and industry overview. Camelid medicine and surgery. Revision 3.71. 1998: 1.1-1.9. Stef JJ, Dodds WJ, Hogan, et al. Intraluminal esophageal manometry: an analysis of variables affecting recording fidelity of peristaltic pressures. Gastroenteology 1974;67: 221-230. Stick JA, Derksen FJ, McNitt DL et al. Equine esophageal pressure profile. Am J Vet Res 1983;44:272-275. 18 Stinton AW, Calhoun ML. Digestive system. In: Dellmann HD, ed. Textbook of Veterinary Histology. Philadelphia:Lea&Febiger, 1976; 207-264. Stinton AW, Calhoun ML. Digestive system. In: Dellmann HD, ed. Textbook of Veterinary Histology, 4th ed. Philadelphia:Lea&Febiger, 1993; 153-193. Twedt DC. Disease of the esophagus. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 4th ed. Philadelphia: WB Saunders, 1995;1 1241142. Vestweber JG, Leipold HW, Knighton RG. Idiopathic megaesophagus in a calf: clinical and pathologic features. J Am Vet Med Assoc 1985;187:1369-1370. Washabau RJ. Canine megaesophagus: pathogenesis and therapy. Proc Am Coil Vet Intern Med 1992;10:671-673. Watrous BJ, Pearson EG, Smith BB, et ai. Megaesophagus in 15 llamas: a retrospective study. J Vet Intern Med 1995;9:92-99. Whitmore I. Oesophageal striated muscle arrangement and histochemical fiber types in guinea-pig, marmoset, macaque, and man. J Anat 1982;134: 685-695. Whitmore I. The ultrastructure of oesophageal striated muscle in guinea-pig and marmoset. Cell Tissue Res 1983;234:365-367. Whitmore I, Notman JA. A quantitative investigation into some ultrastructural characteristics of guinea-pig esophageal striated muscle. J Anat 1987;153:233-239. Won J, Mayer B, Neuhuber WL. Spatial relationships of enteric nerve fibers to vagal motor terminals and the sarcolemma in motor endplates of the rat esophagus: a confocal laser scanning and electron-microscopic study. Cell Tissue Res 1997;287:1 13-118. Worl J, Fischer J, Neuhuber WL. Nonvagal origin of galanin-containing nerve terminals innervating striated muscle fibers of the rat esophagus. Cell tissue research 1998;292:453-461. Yam PS, Shelton GD, Simpson JW. Megaesophagus secondary to acquired myasthenia gravis. J Small Anim Pract 1996;37:179-183. 19 Yoshida Y, Miyazaki T, Hirano M et al. Localization of efferent neurons innervating the pharyngeal constrictor muscles and the cervical esophagus muscle in the cat by means of the horseradish peroxidase method. Neurosci Lett 198 1;22:91-95. Zhou DS, Desaki J, Komuro T. Neuro-muscular junctions of longitudinal and circular muscle fibers of the guinea-pig esophagus and their relation to myenteric plexus. J Auton Nerv Syst 1996;58:63-68. 20 CHAPTER 2. ESOPHAGEAL PHYSIOLOGY OF LLAMAS (Lama glama) ABSTRACT Solid-state esophageal manometry was used to study esophageal physiology in ten normal llamas. Resting pressures and length of the lower esophageal sphincter and upper esophageal sphincter were determined using a station pull-through technique. Pressure events of esophageal peristalsis during a water swallow were recorded throughout the esophagus and the pharynx. The peristaltic parameters determined in the study were amplitude of contraction, duration of contraction, mean rate of pressure change per unit time, and propagation velocity of peristaltic waves. Balloon distention was used to study secondary peristalsis. Propagation velocity of the esophagus when swallowing pelleted feed was determined in three llamas. Natural esophageal activities during experiments were also recorded. Mean (± SD) resting pressures in the upper and lower esophageal sphincters were 8.3 ± 2.8 and 6.7 ± 3.6 mmHg, respectively. The lengths of the upper and lower esophageal sphincters were 4.7 ± 1.2 and 4.5 ± 0.7 cm, respectively. Mean amplitude of contraction was the greatest in the pharynx (169.1 ± 28.4 mmHg) and the upper esophageal sphincter (130.6 ± 39.3 mmHg), and the lowest in the lower esophageal sphincter (34.5 ± 12.4 mmHg). There was no significant difference in 21 mean amplitude of contraction between the cervical (78.7 ± 25.3 mmHg) and thoracic esophagus (68.0 ± 16.9 mmHg). Mean duration of contraction was not significantly different between the cervical (0.40 ± 0.07 s) and thoracic esophagus (0.42 ± 0.09 s), but significantly longer in the lower esophageal sphincter (0.57 ± 0.16 s). Mean rate of pressure change per unit time was the greatest in the pharynx (936.7 ± 203.8 mmHg/s) and upper esophageal sphincter (782.9 ± 353.8 mmHg/s), and the lowest in the lower esophageal sphincter (133.5 ± 41.2 mmHg/s). There was no difference in mean rate of pressure change per unit time between the cervical (395.7 ± 72.2 mmHg/s) and thoracic esophagus (346.2 ± 63.9 mmHgls). There was also no significant difference in the propagation velocity between the cervical (41.9 ± 3.5 cmls) and thoracic esophagus (45.2 ± 3.6 cmls). Propulsive force was generated as soon as the balloon was inflated within the esophagus. Secondary peristalsis was localized to the balloon position. Swallowing was also observed with long duration of balloon inflation. Propagation velocity of grain swallows was 30.2 ± 5.7 cm/s. INTRODUCTION The llama esophagus, like that of the camel other ruminants, is mainly composed of striated muscle throughout the length of the esophagus (Jamdar and Ema, 1982; Fowler, 1998). The most common esophageal problem in llamas is megaesophagus, a term referring to a generalized esophageal dilation. 22 Unfortunately, the overall incidence of the condition is difficult to determine. In an informal survey of veterinarians who routinely treat South American Camelids (SAC's) it was found that most were aware of at least one animal with megaesophagus in their practice. In a retrospective study of 15 llamas with acquired megaesophagus, age of onset ranged from 13 months to 9.5 years (Watrous et al., 1995). Etiology was not determined in 11 of 15 animals. Megaesophagus in one llama was attributed to organophosphate toxicity, vagal neuropathy in another animal and degenerative myopathy in two additional llamas (Watrous et al., 1995). Megaesophagus was reported in a nine year old, male llama with persistent right aortic arch (Butt et al., 2001). A detailed-evaluation of these cases has been hindered by a lack of information about physiologic function of the normal llama esophagus. Accordingly, the objectives of this study were to create a normal esophageal peristaltic profile of the llama esophagus in response to water swallows, to determine the resting pressures and the in vivo length of the upper esophageal sphincter, esophageal body, and lower esophageal sphincter, to study the pattern of the llama esophagus response to balloon distention, and to record the natural occurrence of esophageal activity during experiments such as burps and secondary peristalsis. This study would serve as the baseline for further studies of esophageal dysfunction and megaesophagus in the llama. 23 MATERIALS AND METHODS Animals Ten adult llamas (mean age 5 years, range: 2.8 - 9.3 yr; mean weight 156.7 kg, range: 118.2 - 186.4 kg; 6 females and 4 geldings) from the Camelid Research Herd of the College of Veterinary Medicine at Oregon State University were used for this study. None of these llamas had a history of esophageal problems. All animals were in good physical condition with no evidence of disease. Housing of the animals and the research protocol were approved by the Institutional Animal Care and Use Committee. Animals were fasted overnight before being evaluated. During recordings llamas stood in a llama restraint chute. To avoid possible effects of drug induced alterations on esophageal function, no animals were sedated. All studies were conducted in the large animal hospital in the College of Veterinary Medicine of Oregon State University. Manometric Assembly Esophageal manometry was performed with a 3.7 mm diameter custom made catheter containing four solid-state microtransducers (Model CT/S4L, Medical Measurements Inc., Hackensack, NJ). The microtransducers were placed radially at 90° and at 10 centimeter intervals spanning a total distance of 30 centimeters in the distal end of the two meter long catheter. Length was marked along the catheter in centimeter intervals to the most distal transducer. The catheter was connected via 26 Manometnc assessment of the esophagus in response to balloon distention A different balloon catheter was made from a polyvinyl tube with an inner diameter of 2.2 mm and a length of two meters. Distances were marked in centimeters along the length of the catheter. The latex balloon, five centimeters in length and approximately four centimeters in diameter when it was inflated with 35 milliliters of air, was attached near the closed distal end of the catheter. The proximal end of the catheter was connected to a 60 ml syringe for air injection and to the external transducer (Model P23 ID, Gound Inc., Oxnard, CA) ports to the recorder (Model 5/6H, Gilson Medical Electronics Inc., Middleton, WI). The balloon catheter along with the manometnc catheter was passed via nasal passage into the esophagus. Recordings were done with the steady held at 100 cm to the distal transducer from the nares and at 60 cm. The balloon catheter was positioned such that the balloon was placed at 5, 10, and 20 centimeters distal to the distal microtransducer (the 4th microtransducer), halfway between the middle microtransducers (the 2nd and 3( microtransducers), and 5, 10, 20 centimeters proximal to the proximal microtransducer (the 1st microtranducer). Five transient (one second) and five sustained (five seconds) balloon inflations were used at each station. At least 30 seconds rest separated balloon inflations. During balloon inflation both catheters were held in a fixed position, and were not allowed to move distally. The results were recorded at a paper speed of 25 mmlsec during balloon inflation and one mmlsec during rest. Propagation force, a detectable pull on the 27 catheter assembly, was noted. During each experiment natural occurrences of esophageal activity such as burps and secondary peristalsis were also recorded. Propagation velocity of the esophagus when swallowing pelleted feed Propagation velocity of the esophagus responding to swallows of pelleted feed was determined in 3 llamas using another water filled balloon catheter. The catheter was made from a plastic tube (2.2 mm internal diameter) with two small balloons separated by 50 cm. Fifteen swallows were recorded at 100 cm from the nares to the distal balloon. Data and Statistical Analysis Length of physiologic esophageal regions The in vivo length of each esophageal region was measured in centimeters by subtracting the distances visible at the nares when the microtransducer entered into and out of the region as determined by physiologic function. For the lower esophageal sphincter, the indicator was a high pressure zone between Cl and the distal esophagus. The thoracic esophagus was indicated by negative pressure in the thorax and fluctuation of resting pressure during respiration. In the cervical esophagus, fluctuation of resting pressure during respiration disappeared and resting pressure was essentially equal to reference atmospheric pressure. For the upper esophageal sphincter, there was a high pressure zone between the proximal 28 end of the esophagus and the pharynx. The pharynx identified when resting pressure returned to reference atmospheric pressure. Resting pressures Resting pressure measurement at each station was calculated from the mean of the pressures measured at all four microtransducers at that station. Resting lower esophageal sphincter pressure was measured by subtracting the baseline of the thoracic esophageal pressure from the mean of peak pressures. Resting upper esophageal sphincter pressure was measured by subtracting the baseline of cervical esophageal pressure from the mean of peak pressures. Peristaltic parameters Measurement was made of the following peristaltic parameters: amplitude of contraction, duration of contraction, mean rate of pressure change per unit time during contraction, and propagation velocity of the peristaltic wave (Figure 2.1). Amplitude (mmHg) was measured from the baseline to the contraction peak. Duration (s) was the interval from the initial upstroke of peristaltic contraction until the return to baseline at a given recording site. Mean rate of pressure change per unit time (mmHg/s) was calculated as amplitude of contraction divided by one half of the duration of associated contraction. Propagation velocity (cmls) was measured by identifying the interval between the initial upstroke of the peristaltic contraction at adjacent recording sites. For each parameter there were five recordings at each of 29 the four transducers for each location. All measurements of each parameter from the four transducers were used for calculation and creating the profile plot for each location. A total of measurements was made at each site. A physiologic profile plot was created for each parameter and for each animal. As each animal had a different anatomic esophageal length, to create an overall profile esophageal length was normalized and data was plotted as a percentage of the total esophageal length. 0% represented the proximal end and 100% represented the distal end of the esophagus. 30 Figure 2.1 A representative tracing demonstrating calculation of peristaltic parameters. Amplitude (A) is calculated from baseline to peak. Duration (D) is calculated as the excursion time from baseline and returning to baseline following a contraction. Mean rate of pressure change per unit time (dP/dT) is amplitude divided by ½ of duration and velocity (V) is measured as the time between the beginning of two adjacent contractions. 31 Statistical analysis All values are expressed as mean ± SD. As peristaltic velocity was only deteiiiiined for cervical and thoracic esophagus it was tested with paired-t test. The other esophageal parameters (amplitude of contraction, duration of contraction, and mean rate of pressure change per unit time) were determined for all four regions and thus were tested with repeated measures ANOVA and with Tukey's Studentized Range (HSD) test for multiple comparisons of means. All statistical analyses were performed with the SAS system for Windows software program, Version 8 (SAS Institute mc, Cary, NC, USA) with a two-tailed significance criterion set atp = 0.05. RESULTS The llama esophagus had 4 functionally different regions: the pharyngoesophageal segment including the upper esophageal sphincter, the cervical segment, the thoracic segment, and the lower esophageal sphincter. The mean resting pressure (± SD) of the upper esophageal sphincter, cervical portion, thoracic portion, and lower esophageal sphincter was 8.3 1±2.76, 0, -5, and 6.67±3.64 mm}Ig respectively (Table 2.1). The in vivo length of the upper esophageal sphincter, cervical portion, thoracic portion, and lower esophageal sphincter was 4.7±1.16, 64.3±1.48, 37.8±3.16, and 4.5±0.71 centimeters respectively (Table 2.1). The amplitude of contraction was highest in the pharyngoesophageal region (169.1±28.4, 130.6±39.3 mmHg for the pharynx and upper esophageal sphincter 33 300 C) I 250E E 200C.) 0 150- C.) 0 100- 0 -10 -5 0 5 -I I I I I I I I I . 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 Percent of esophageal length Figure 2.2 Box and whisker plot of the amplitude of contraction (mniHg) of the esophagus during water swallows of ten llamas. Note that the amplitude of contraction was highest in the pharyngoesophageal region and was lowest in the lower esophageal sphincter. 34 '4- 0 -10 -5 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 Percent of esophageal length Figure 2.3 Box and whisker plot of the duration of contraction (s) of the esophagus during water swallows of ten llamas. Note that the duration of contraction was highest in the lower esophageal sphincter. 35 C') I E 2000a) 1750E 1500- 0 C) 12501000- Cu C.) 750500- 0 Cu 250- 0I Cu I -10 -5 I I I 0 5 10 I I I I I I I I I I I I I I I I I I 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 100 a) Percent of esophageal length Figure 2.4 Box and whisker plot of mean rate of pressure change per unit time (mmHg/s) of the esophagus during water swallows from ten llamas. Note that the mean rate of pressure change per unit time was highest in the pharyngoesophageal region and lowest in the lower esophageal sphincter. 36 80 0 70- 0000 60- I I I 0 0 'Ii' ±TIhT ITTT T 20- -5 0 5 10 15 20 25 30 35 40 45 50 55 60 65 70 75 80 85 90 95 Percent of esophageal length Figure 2.5 Box and whisker plot of propagation velocity (cmls) of the esophagus during water swallows of ten llamas. Note that the propagation velocity is almost constant throughout the esophagus except in the pharyngoesophageal region, where it was lowest. This does not include lower esophageal sphincter. Evaluation of the physiologic data led to a division of the esophagus into upper esophageal sphincter, esophageal body including cervical and thoracic portions, and lower esophageal sphincter (summarized in Table 2.1) 37 Table 2.1 Results of manometric evaluation in ten normal llamas. Note that resting upper esophageal sphincter pressure was markedly asymmetric; the value shown was the average from all four channels. The percent of swallows propagated into the esophageal body was 100%. Pharynx UES Esophageal body Regions LES Cervical Thoracic Total 30.1± 34.4± 98.6± NA 1.5 1.4 1.9 136.5 ± 3.7 4.7± 64.3± 1.5 37.8± 3.2 102.1± 4.0 4.5± 1.2 0 8.3± 2.8 0 -5 NA 6.7± 3.6 169.1± 28.4 78.7± 25.3 68.0± NA 16.9 34.5± 12.4 A 130.6 ± 39.3 B C C D Duration of peristaltic contraction (s) 0.38± 0.05 0.37± 0.08 0.40± 0.07 0.42± 0.09 Comparison of mean (Tukey's group)* A A A A Paramete'NNNN Distance from nares to proximal margin of each region (cm) Length of region (cm) Resting pressure (mmHg) Amplitude of peristaltic contraction (mmHg) NA NA 0.7 Comparison of mean (Tukey's group)* NA 0.57± 0.16 B 38 Table 2.1 (Continued) Mean rate of pressure change per unit time dP/dT (mmHg/s) Comparison of mean (Tukey's group)* Propagation velocity (cmls) p-value 395.7 ± 72.2 346.2± 63.9 203.8 782.9 ± 353.8 A A B B NA 41.88± 3.46 45.18± 3.57 0.064 0.064 936.7 ± NA 133.5 ± 41.2 C C - NA NA NA * For each comparison, means of the same letter are not significantly different. NA = Not Applicable The response of the llama esophagus to balloon distention is summarized in Table 2.2. The amplitude of contraction was higher when the balloon was close to the microtransducer than when the balloon was farther away. The esophageal propulsive force was generated as soon as the balloon was inflated. The propulsive force with sustained balloon distention varied among llamas and within a llama. There were two types of propulsive force: (1) sustained propulsive force which gave a sustained pull as long as the balloon was inflated, and (2) phasic propulsive force which gave two to four short pulls during sustained balloon inflation. Sometimes, one to two swallows also occurred during sustained balloon inflation. 40 IkdtIdI,ILIldgl.,gIII.1t.I.dIIlrjI.lhdI ,i,i..j11i1g tu'IIiiI11IHi ll1IliFiiIH 11IUI1111I11 Tracing .iu '!!! IuituIIISIlruhIu, 11p111111 1111! 'I'1! llhiiflhIiiUII JlI'uHlIt niilllli'LIllI IU i}tflilh!II1IUQ! IL IHuillI 1111 Iii h11IUIIIhIII!IH 'JIlIIil!flhi1Ii I; - Tracing 2 Tracing 3 IIllItIIIIUIIllUhIIuI Ill!IIIHJI III! lluItllHfl__ Iit,illII IIfliiIiiUIIi 111H IliIIHl'llll IiiIUIiIhIIllItIIIflflll UI;IIHUiUhIIilIuhh IhlwIilI1llHllUUp1l1 IIh!!HI 11!UtflUIII!II lhitIIHIII11ulHlluiiiueU IIIUHIWIIIIOUh llhIIIIIifliI,!UUflllhI I1IIiiI1i'!IIIIIIIIJIflI UI IDUL mu i Tracing 4 mu IiUiMIIIUI 4hj1uq1 " I''.' t'IIIiII ILIII iItIII'U II 1iI 10K huIIUUICii I UI I III III HPaII L1IH IIIUU'Uu IQII}I!H 111111 IiiViIltil I H he I'a e15'111(IUI1IPU iU!iH'_HI FIiIdLIHi i dilli iH1 III Ufl II III1!III II UilHHHldIIUIdUWU1UHllUh;iHHilllillHUiHHIIhifl iflUflhI IIIII}IIIiiIIHtIPIUItHIWIIIIHIIIflil II1IIIUjIHUi!II!UHHUIIIP IUHhIIlil lUll 11!!I lUi!IrnlH 111111 ilii !hi IIIilH hUll 19111111111111 illthUIiIHhiIflHflIlU1111 111111111111 ulli I. mIlmil IllhtuhIlliHUhl!IhillllHbtIflhlliH 111111111111 litlill Uliluuti 111 1111111 111 IlillillhllIl' lliiilHUI_lIiIiHIH11 111111 11111111 hut uflllP11Ifl1iHuIUil tilt eli h'iI 11tH Ui 11li'l II 111111 I I H' illill Figure 2.6 Cl contraction generating a burp into the esophagus. The distal two tracings (tracing 3 and 4) were placed in Cl region and the proximal two tracings (tracing 1 and 2) were placed in the distal thoracic esophagus. Three Cl contractions were shown but only the middle contraction of Cl (arrows) generated a burp into the esophagus (bent-up arrows). 41 Swallow marker Tracina I Tracing 2 F'- Tracing 3 1,!Q!! ii;;;z 1 1L lUll !IIiU!I !!lUhiII lllIl9HIPllllPMqllIHl! I1 IIIIIHIOIIIIIIIII;Ill P!Ji Trac ng 4 II B Lll 'In Ui 1I'H lli,lliilL d'iilll1H Li'I IIL.- ' ; r "ii b 2 iriI IIIII1KUI t1l,,ht ii 'hil lI,Uh, th!MI!!lUhIfl!IUli!H iliW !E.lU 'I1IlH!iUflhlU H HftII t'tbulI!!!H II!h'iH HI UUI1hiiH ;111hi' "I"IJH ii1j1j1 bi I r II UI .: UU.UIMIUI Allil fljI HUlitluul .FUhIUllhi1W.i'JIl ufflI - Figure 2.7 Secondary peristalsis occurring immediately after a burp in the thoracic portion of the esophagus. Tracing 3 and 4 were placed on the proximal thoracic esophagus; tracing 1 and 2 were placed on the distal cervical esophagus. The swallow marker was placed in the hypopharynx. Three esophageal motor activity including a burp, primary and secondary peristalsis occurred. A burp (arrows) moved upward throughout the esophagus. Secondary peristalsis (bent-up arrows) occurred immediately after a burp and moved aborally starting at the level of thoracic esophagus to keep the esophagus clean. Primary esophageal peristalsis (curved right arrows) associated with pharyngeal contraction (star) was an additional mechanism and also moved aborally to make sure the esophagus was clean. 42 Swallnw marker Tracing I r Tracing 2 Tracing 3 Tracing 4 Figure 2.8 Deglutitive inhibition; with a series of swallows (arrows) at short intervals, the esophagus remained inhibited, then a normal peristaltic contraction occurred after the last swallow in the series. 43 DISCUSSION Esophageal manometry, a technique to study motor activity of the esophagus by measuring intraluminal esophageal pressure has proved to be a useful tool in both the clinical and basic research settings. Studies performed in a variety of species, including humans, primates, cats, dogs, and opossums, have provided information on esophageal function in normal and disease states (Castell and Gideon, 1999). Esophageal manometry has been used in large domestic species such as sheep (Can et al., 1983; Clark et al., 1987), cows (Stevens and Sellers, 1960; Clark et al., 1987), and horses (Stick et al., 1983; Clark et al., 1987) but not previously in the llama. A manometric apparatus consists of a pressure sensor and transducer combination that detects the esophageal pressure complex and transduces it into an electrical signal sent to a recording device to amplify, record, and store that electrical signal. Accurate recording of intraluminal esophageal pressure is important for satisfactory examination of esophageal motor function. Although each component can potentially affect recording fidelity, most attention is rightfully focused on the pressure sensor and transducer combination. Recorders, whether they are ink writing polygraphs, thermal writing polygraphs, or computers with analog to digital converters, all possess response characteristics far in excess of that required for recording esophageal pressure complexes (Kahrilas et al., 1994). There are two main types of esophageal manometric catheters in terms of the pressure sensor and transducer components of a manometric assembly: water-perfused 44 catheters with volume displacement transducers and strain gauge transducers with solid-state circuitry (Kahrilas et al., 1994). In the water-perfused catheter systems, the catheter diameter, catheter length, and water infusion rate are variables that can affect recording fidelity. The major cause of recording error is "failure" of fluid delivery by the infusion pump during a pressure transient (Stef et al., 1974). A solid-state catheter has the advantage that, unlike the water infusion system, pressures are measured directly and are unrelated to the relative position of the measured animal and the equipment; therefore, studies may be performed with the animal in any position. This is especially important given the unusually long neck of the llama. In addition, the response time of the solid-state catheter is much faster than that of the water infusion system, making possible more accurate measurements of the pressures generated by the esophageal striated muscle (Kahnlas et al., 1994; Castell and Gideon, 1999). In preliminary studies in our laboratory the water-infusion catheter was inadequate. Thus the solid state catheter was used for these studies. Esophageal manometry has been used to determine the physiological length of the portions of the esophagus in the human (Li et al., 1994). In the llama, the length of the cervical esophagus (64.3±1.49 cm) is almost twice that of the thoracic esophagus (37.8±3.16 cm) in keeping with the long neck of the llama. This proportion is slightly larger than that of the horse that has a cervical and a thoracic length of approximately 70cm and 50 cm respectively (Murray, 1998) but differs from the shorter necked the cow (the proportion is approximately 1; the total 45 esophageal length is approximately 90-95 cm long, the cervical portion is approximately 42-45 cm long, the thoracic portion is approximately 48-50 cm (Schummer et al., 1979), and the sheep with the total esophageal length approximately 45 cm long (Getty, 1975). The length of the upper esophageal sphincter in the llama (4.7 cm) is similar to that of the horse (4.3 cm), but is longer than that of the larger cow (3.6 cm), the smaller sheep (2.9 cm) (Clark et al., 1987), and the human (1 cm) (Kahrilas et al., 1988). The length of the lower esophageal sphincter in the llama (4.5 cm) is similar to that of the sheep (4.3 cm) and the human (3-4 cm) (Kahrilas et al., 1994), but is shorter than that of the cow (5.7 cm) and the horse (10.6 cm) (Clark et al., 1987). The resting pressure of the upper esophageal sphincter in the llama (8 mmHg) is remarkably low when compared to other species: the horse (85 mmHg), the cow (82 mmHg), the sheep (36 mmHg) (Clark et al., 1987) and the human (84 mmHg) (Castell et al., 1990). The high upper esophageal sphincter resting pressure is helpful to prevent the acid reflux into the mouth of the human (Kahrilas et al., 1999). In the llama, the low resting upper esophageal sphincter pressure may facilitate movement of gas from burps out of the esophagus and ease the regurgitated bolus into the mouth for rechewing and swallowing. Burps are associated with Cl contraction. The overall pattern of llama forestomach motility appears quite different from that in the ruminant in that there is a much greater frequency of forestomach contractions (Vallenas and Stevens, 1971; Heller et al., 1984). Thus, burps may occur more frequently in the llama and may require lower 46 upper esophageal sphincter resting pressure than that of the ruminant. The upper esophageal sphincter resting pressure in the llama is also markedly asymmetric, similar to the human (Kahrilas et al., 1987; Welch et al., 1979). The asymmetry is understandable in view of the noncircular or slitlike configuration of the sphincter. The asymmetry disappears due to altered morphology following laryngectomy in the human (Welch et al., 1979). The lower esophageal sphincter resting pressure in the llama (7 mmHg) is slightly lower than that of the sheep (lOmmHg), and the horse (12.7 mmHg), and is lower than that of the cow (20.5 mmHg) (Clark et al., 1987), and the human (29 mmHg) (Richter et al., 1987). In the human, the lower esophageal sphincter is the antireflux barrier designed to limit the frequency and volume of contact between gastric contents and the esophageal epithelium (Orlando, 1999). Amplitude of contraction in the pharynx of the llama (169 mmHg) is greater than that of the human (122 mniHg) (Castell and Gideon, 1999). Amplitude of contraction in the upper esophageal sphincter of the llama (131 nmiHg) is less than that of the horse (208 mmHg) and the cow (238 mmHg), but is greater than that of the sheep (106 mmHg) (Clark et al., 1987). Amplitude of contraction in the cervical portion (79 mmHg) and thoracic portion (68 mmHg) of the llama is less than that of the esophageal body of the horse (approximately 96 mmHg) and that of the esophageal body of the cow (approximately 110 mmHg), but greater than that of the esophageal body of the sheep (47 mmHg) (Clark et al., 1987). 47 Duration of contraction of water swallows in the thoracic esophagus of the llama (0.41 second) is similar to that saliva swallows in the caudal thoracic esophagus of the sheep (approximately 0.48 second) (Can et al., 1983). The duration in the llama is longer in the most distal segment of the esophagus, including the lower esophageal sphincter (0.57 second), but it is still very short when compared to the distal segment of the human esophagus, which consists of smooth muscle (4 seconds) (Kahnlas et al., 1994), and the caudal third of the esophageal body of the horse which also consists of smooth muscle (6.3 seconds) (Clark et al., 1987). The difference in the duration between the most distal segment and the rest of the esophageal body in the llama may reflect the difference in striated muscle type proportion. The proportion of type 1 fibers (slow contraction fibers) increases markedly in the distal thoracic esophagus (chapter 3). Mean rate of pressure change per unit time in the pharynx (937 mmHglsecond) and UES (783 mmHg/second) of the llama is greater than that of the esophageal body of the llama (37 lmmHglsecond) and the pharynx of the human (443 mmHglsecond) (Castell and Gideon, 1999). The reason that the mean rate in the pharynx and UES is higher than that of the esophageal body may be due to the greater thickness of the muscular wall in the pharyngoesophageal region. The decreased mean rate in the most distal esophagus, including LES, compared to rest of the esophageal body may reflect the difference in the proportion of striated muscle fiber types. The proportion of type 1 fibers (slow contraction) increases markedly in the distal thoracic esophagus (chapter 3). 49 i.e., gulping air (Hollis and Castell, 1975; Richter et al., 1987). The propagation velocity of the solid swallows (30 cmlsec) is lower than that of the water swallows (43 cmlsec) in these llamas, similar to the previous studies in the human (Johnston et al., 1993; Keren and Argaman Golan, 1992; Dooley et al., 1990). Earlier studies have suggested that the duration of peristaltic contraction after wet swallows is greater than after dry swallows (Dodds et al., 1973; Hollis and Castell, 1975), but in more recent studies there was no significant different in the duration between these types of swallows (Kaye and Wexler, 1981; Richter et al., 1987). The duration of contraction increases, however, in an aboral direction in the human (Richter et al., 1987; Goyal and Sivarao, 1999). Larger bolus volumes elicit stronger peristaltic contractions in the dog (Janssens et al., 1973). The amplitude of penstalsis ensures that it completely sweeps the bolus into the stomach without leaving any residue behind. However, weaker contractions can leave some residue behind. Food residue left behind in the esophagus by ineffective primary peristalsis is removed by the secondary peristalsis. Primary peristalsis in the striated muscle portion of the esophagus is mediated by sequential vagal excitation arising in the brainstem (Diamant, 1997). Andrew (1956) suggested that sequential discharge of the motor neurons destined for progressively more distal levels was responsible for penstalsis in the rat. Roman and Gonella (1987) performed experiments in which the central portion of a sectioned vagus (containing nerve fibers from lower motor neurons to the striated muscle portion of the esophagus) was sutured to the distal end of the motor nerve 50 innervating the stemocleidomastoid and trapezius muscles, allowing vagal motor fibers to reinnervate these muscles. They then recorded EMG activity in muscle units in the neck in response to swallowing. It was found that activation of deglutition induced sequential contraction of the reinnervated muscles and that this coincided with esophageal peristaltic contractions. This observation further supported the view that sequential activation of vagal motor neurons elicited peristaltic activity. Thus, the difference in the propagation velocity in the llama and in the sheep may be attributed to a difference in the rate of sequential discharge of the motor neurons responsible for esophageal peristalsis in these species. Normally, the esophagus responds in a one-to-one manner to each pharyngeal swallow. However, the time required for the pharyngeal stage of swallowing is much shorter than that for the esophageal stage. During periods of rapid successive swallowing, esophageal activity is inhibited and only the last swallow of the swallowing train is associated with esophageal peristaltic contraction (Ask and Tibbling, 1980). This phenomenon is called deglutitive inhibition. Sifrim et al. (1992) demonstrated a wave of inhibition prior to peristaltic contraction motility in the human. Deglutitive inhibition is critically important for the passage of swallowed food through the esophagus, and failure of deglutitive inhibition is associated with esophageal motility disorders (Sifrim et al., 1992). Deglutitive inhibition could be demonstrated in the llamas by infusing continuously larger volumes of water. This stimulated rapid successive swallows and only the last swallow could initiate esophageal peristalsis. 51 Secondary peristalsis is distinguished from primary peristalsis because it is localized to the esophagus and is not accompanied by pharyngeal or upper esophageal contraction. Experimentally, secondary penstalsis is elicited by transient esophageal distention and deflation of an intraluminal balloon (Paterson et al., 1988). Esophageal distention may also induce primary peristalsis (Goyal and Sivarao, 1999). Winship and Zboralske (1967) reported that if a balloon is inflated in the human esophageal body and prevented from moving distally, an aborally directed steady force of up to 200 g is exerted on the balloon, called the esophageal propulsive force. The force develops immediately upon inflation of the balloon, and the force is sustained as long as the balloon is inflated. The esophageal propulsive force appears to increase with increasing bolus size (Williams et al., 1993). During the period of fixed balloon distention, no contractions occur in the esophagus distal to the balloon; however, when restraints are removed from the balloon, the contraction producing the localized propulsive force is converted to a peristaltic sequence that progresses distally, pushing the balloon ahead of it (Winship and Zboralske, 1967). This propulsive force is produced by phasic and tonic contractions of the circular and longitudinal muscles at and just above the balloon. These consist of simultaneous contractions that become multipeaked, repetitive, and associated with a sustained rise in the basal pressure with increasing distention volumes. Distention-induced peristalsis in the striated muscle segment of the dog and sheep esophagus is entirely dependent on central vagal pathways. Thus, there is no difference between primary and secondary penstalsis in the striated muscle 52 segments other than in the method of initiation and in the fact that the occurrence of the latter is independent of oropharyngeal component (Goyal and Sivarao, 1999). Esophageal response to balloon distention in the llama is as described above. The response is local to the balloon site, propulsive force is generated when the balloon is inflated and fixed in place, and swallows usually occur during sustained balloon distention in an attempt to pull the balloon down. Further, secondary peristalsis in the thoracic esophagus, which lies horizontally and connects to Cl, usually occurs after a burp, which is generated from llama forestomach contraction. Natural occurrence of secondary peristalsis in the cervical esophagus was rare during the experiment. This could be explained by the vertical nature of the cervical esophagus and the location of the cervical esophagus far away from Cl. There is little chance of Cl fluid content bouncing back during forestomach contraction and burp to reach the cervical portion of the esophagus and stimulating secondary penstalsis. Thus, in general the contraction of the thoracic esophagus occurs more frequently than that of the cervical esophagus. This physiologic finding correlates with anatomic findings; the proportion of type 1 muscle fibers is markedly increased in the thoracic esophagus (chapter 3). Type 1 muscle fibers, which are resistant to fatigue, may be required to perform the busy function in the thoracic portion of the esophagus. Physiologically, the llama esophagus is more similar to the ruminant esophagus in that it consists of striated muscle throughout than to the horse esophagus, which consists of both striated and smooth muscles. As described the characteristics of 53 esophageal penstalsis in the llama are short in terms of duration of contraction and fast in terms of propagation velocity. Although the llama esophagus is long, with the velocity approximately ten times greater than that of the human it takes only a few seconds for the peristaltic wave to travel from the proximal end to the distal end of the llama esophagus. The llama esophagus also possesses important nerve reflexes such as deglutitive inhibition and secondary peristalsis similar to, other mammalian species. REFERENCES Andrew BL. The nervous control of cervical esophagus of the rat. J Physiol 1956; 134:729-740. Ask P, Tibbling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol 1 980;238 :G485-G490. Butt TD, Macdonald DG, Crawford WH. Persistent right aortic arch in a mature llama. Vet Rec 2001;148:118-119. Can DH, Scott PC, Titchen DA. Manometric and electromyographic observations of the oesophagus of sheep in eructation, regurgitation and swallowing. Quarterly Journal of Experimental Physiology 1983 ;68 :661-674. Castell JA, Dalton CB, Castell DO. Pharyngeal and upper esophageal sphincter manometry in humans. Am J Physiol 1990;258:G173-G178. Castell JA, Gideon RM. Esophageal manometry. In Castell DO, Richter JE, eds. The Esophagus, 3'' ed. Philadelphia: Lippincott Williams & Wilkins, 1999;101117. Clark ES, Morris DD, Whitlock RH. Esophageal manometry in horses, cows, and sheep during deglutition. Am J Vet Res 1987;48:547-551. Diamant NE. Neuromuscular mechanisms of primary peristalsis. Am J Med 1997; 103 :40s-43s. 54 Dodds WJ et al. A comparison between primary esophageal penstalsis following wet and dry swallows. J Appl Physiol 1973;35:851-857. Dooley CP, Di Lorezo C, Valenzuela JE. Esophageal function in humans. Effect of bolus consistency and temperature. Dig Dis Sci 1990;35:167-172. Fowler ME. Digestive system. In: Medicine and Surgery of South American Camelids: Llama, Alpaca, Vicuna, Guanaco. 2nd ed. Iowa: Iowa State University Press, 1998;305-359. Getty R. Digestive system of the sheep. In: Sisson and Grossman's the Anatomy of the Domestic Animals. 5th ed. Philadelphia: W.B. Saunders Company, 1975;477484. Gidda JS, Buyniski JP. Swallow-evoked peristalsis in opossum esophagus: role of cholinergic mechanisms. Am J Physiol 1986;251:G779-G785. Goyal RK, Sivarao DV. Functional anatomy and physiology of swallowing and esophageal motility. In: Castell DO, Richter JE, eds. The Esophagus, 3Id ed. Philadelphia: Lippincott Williams & Wilkins, 1999;1-32. Heller R, Gregory PC, Engelhardt. Pattern of motility and flow of digesta in the forestomach of the llama (lama guanacoef glaina). J Comp Physiol B 1984; 154:529-533. Herwitz AL, Duranceau A, Haddad JK. Esophageal manometric techniques. In:Smith LH, ed. Major Problems in Internal Medicine. Philadelphia: WB Saunders Co, 1979;27-33. Hollis JB, Castell DO. Effect of dry swallows and wet swallows of different volumes on esophageal peristalsis. J Appi Physiol 1975;38:1161-1164. Jamdar MN, Ema AN. The submucosal glands and the orientation of the musculature in the oesophagus of the camel. J Anat 1982;135:165-171. Janssens J, Valembois P, Vantrappen G, et al. Is the primary peristaltic contraction of the canine esophagus bolus-dependent? Gastroenterology 1973 ;65 :750-756. Johnston BT Collins JSA, McFarland RJ, et al. A comparison of esophageal motility in response to bread swallows and water swallows. Am J gastroenterol 1993 ;88:35 1-355. 55 Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology 1994;107: 1865-1884. Kahrilas PJ, Dent J, Dodds WJ et al. A method for continuous monitoring of upper esophageal sphincter pressure. Dig Dis Sci 1987;32:121-128. Kaye MD, Wexler RM. Alteration of esophageal peristalsis by body position. Dig Dis Sci 198 1;26:897-901. Keren S, Argaman Golan M. Solid swallowing versus water swallowing: manometric study of dysphagia. Dig Dis Sci 1992;37:603-608. Li Q, Castell JA, Castell DO. Manometric determination of esophageal length. Am J Gastroenterol 1994;89:722-725. Murray MJ. The esophagus. In: Reed SM, Bayly WM, eds. Equine Internal Medicine. Philadelphia: WB Saunders Co, 1998;608. Orlando RC. Pathophysiology of gastroesophageal reflux disease. In Castell DO, Richter JE, eds. The Esophagus, 3rd ed. Philadelphia: Lippincott Willams &Wilkins, 1999;409-419. Paterson WG, Rattan 5, Goyal RK. Esophageal response to transient and sustained esophageal distention. Am J Physiol 1988;255:G587. Richter JE, Wu WC, Johns DN et al. Esophageal manometry in 95 healthy adult volunteers. Dig Dis Sci 1987;32:583-592. Roman C, Gonella J. Extrinsic control of digestive tract motility. In Johnson LR, ed. Physiology of the Gastrointestinal Tract 2nd ed. New York: Raven Press, 1987:507. Schummer A, Nickel R, Sack WO. The alimentary canal. In: The Viscera of Domestic Mammals, New York: Springer-Verlag, 1979;99-202. Sears VW Jr, Castell JA, Castell DO. Comparison of effects of upright versus supine body position and liquid versus solid bolus on esophageal pressures in normal humans. Dig Dis Sci 1990;35:857. Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary penstaltic contractions in the human esophagus. Gastroenterology 1992;103:876882. 56 Stef JJ, Dodds WJ, Hogan, et al. Intraluminal esophageal manometry: an analysis of variables affecting recording fidelity of peristaltic pressures. Gastroenterology 1974;67: 221-230. Stevens CE, Sellers AF. Pressure events in the bovine esophagus and reticulorumen associated with eructation. Am J Physiol 1960;199:598-602. Stick JA, Derksen FJ, McNitt DL et al. Equine esophageal pressure profile. Am J Vet Res 1983;44:272-275. Vallenas AP, Stevens CE. Motility of the llama and guanaco stomach. Am J Physiol 1971 ;220:275-282. Watrous BJ, Pearson EG, Smith BB, et al. Megaesophagus in 15 llamas: a retrospective study. J Vet Intern Med 1995;9:92-99. Welch RW, Luckmann K, Ricks PM et al. Manometry of the normal upper esophageal sphincter and its alterations in laryngectomy. J Clin Invest 1979;63:1036-1041. Williams D, Thompson DG, Heggie L, et al. Responses of the human esophagus to experimental intraluminal distention. Am J Physiol 1993;265:G196-G203. Winship DH, Zboralske FF'. The esophageal propulsive force: esophageal response to acute obstruction. J Clin Invest 1967;46:1391-1401. 57 CHAPTER 3. A GROSS, HISTOLOGIC, MuSCLE HISTOCHEMICAL, AND ULTRASTRUCTURAL STUDY OF TIlE ANATOMY OF THE LLAMA ESOPHAGUS ABSTRACT The length and diameter of the esophagus in 25 normal llamas was measured. Ten of these llamas that had been examined by esophageal manometry were used for more complete gross anatomic and microanatomic studies including histology, muscle histochemistry and ultrastructure. Cervical, thoracic, and total lengths of the esophagus in all animals were determined. Diameter of the esophagus was also determined in the cranial cervical, middle cervical, thoracic inlet, middle thoracic, and caudal thoracic regions. General histologic findings were recorded and compared with other species. Muscle fiber type classification was determined with myosin ATPase staining. Ultrastructure of the esophageal striated muscle was studied by transmission electron microscopy. Mean (± SD) of the total length of the esophagus was 122±7 cm with the cervical length (80 ± 4 cm) and thoracic length (42 ± 4 cm). Mean of the outer diameter of the esophagus ranged from 2.5 ± 0.3 cm in the cranial cervical to 3.9 ± 0.8 cm in the caudal thoracic region. The mucousal epithelium was a keratinized, stratified squamous epithelium throughout the esophagus. The submucosal glands were abundant throughout the esophagus. The two layers of the tunica muscularis consisted of striated muscle throughout the esophagus. The orientation of the muscle fibers was mixed. Type 2 muscle fibers predominated throughout the 58 esophagus. Type 1 muscle fibers were also present and gradually increased from cranial (1%) to the caudal portion (30%) of the esophagus. Ultrastructure of the esophageal striated muscle was similar to that of general skeletal muscle. INTRODUCTION The anatomy of the normal esophagus has been well described in humans, rodents, and animals used as the models for humans such as the opossum and monkey. On the other hand, there has been little research on the esophageal morphology in domestic large animal species e.g., cows, sheep, horses, donkeys, llamas, alpacas, and camels. Those studies that have been done in various species demonstrated anatomical variation in the esophagus. For example, the tunica muscularis in ruminants is composed entirely of striated muscle along the whole length of the esophagus (Schummer et al., 1979), but only the proximal 5% of the human esophagus is entirely striated muscle (Meyer et al., 1986). Other aspects for comparison are the presence or absence of keratinized epithelium, the presence, absence, and distribution of muscularis mucosae, myenteric plexus, and submucosal glands. The anatomical differences among species may reflect the phylogenetic adaptation for different foodstuffs consumed by the different species. In general, the camelid esophagus is similar to that of ruminants (Fowler, 1998). There are, however, some differences between the camel (dromedary) esophagus and ruminant esophagus. For example, the submucosal glands are well developed throughout the camel esophagus, but are found only in the pharyngo-esophageal 59 region in ruminants (Jamdar and Ema, 1982; Schummer et al., 1979). In general, the muscularis mucosa occurs throughout the length of the esophagus but is incomplete in the ruminants (Deilman and Brown, 1976). The muscularis mucosae in the camel is present in the form of a few scattered strands of smooth muscle in the caudal esophagus (Jamdar and Ema, 1982). The anatomy of the llama esophagus has not been studied in detail. One of the principal diseases of the llama esophagus is megaesophagus. Though the incidence of megaesophagus is low, the individual animal that is affected suffers a long, gradual decline in health. In most cases, etiology of megaesophagus of the llama is unknown (Watrous et al., 1995). The objectives of this study, therefore, were to describe the normal esophagus of the llama in terms of gross and microscopic anatomy, including muscle fiber type and ultrastructure with an emphasis on the musculature. This study would serve as the baseline for study of megaesophagus in the llama. MATERIALS AND METHODS Animals Twenty-five adult llamas (mean age 6.5 years, range 2 to 18 years; mean weight 147.3 kilograms, range 98.2 kilograms to 186.4; 21 females and 4 males) from Camelid Research Herd of the College of Veterinary Medicine at Oregon State University were used for study of the gross anatomy of the llama esophagus. From this number, ten normal llamas (6 females and 4 males) that had been examined by 60 esophageal manometry were used for the microanatoniic studies. Housing of the animals and the research protocol were approved by the Institutional Animal Care and Use Committee. Gross Anatomy The llama was humanely euthanized with intravenous pentobarbital at 0.45m1/kg body weight. The esophagus was exposed and the course of the esophagus was observed along the entire length. The cervical, thoracic, and total lengths of the esophagus were measured in situ in centimeter. The outer diameter of esophagus was measured in situ in centimeter at five levels: (1) cranial cervical, (2) middle cervical, (3) thoracic inlet, (4) middle thoracic, and (5) caudal thoracic. After each level was marked, the esophagus was removed intact from the body. Histologic Anatomy A section, approximately 5 cm in length, was taken from the esophagus at each level as previously described and placed in 10% buffered formalin. Each sample was trimmed, embedded in paraffin, sectioned with sharp steel knives at 5 tm, stained with H&E and examined under the light microscope. The general appearance was observed and noted. In each section, the average thickness of the tunica muscularis was measured in mm at four positions with a 90° separation around the tubular esophageal section using a calibrated ocular micrometer. The lobules of submucosal glands were also counted at each level of the esophagus. 61 Each lobule was defined as completely surrounded with connective tissue such that some lobules would be larger and some would be smaller. Muscle Histochemical Study A fresh specimen, approximately 1.5 x 1.5 cm2, was excised using a sharp razor blade and removed from each region along the length of the esophagus. The specimen was transferred to a gauze moistured with cold saline solution. The specimen was then held in a petri dish on ice awaiting freezing. The specimen was trimmed and the mucosa removed. The specimen was viewed under a microscope to determine the orientation of muscle fibers so that mounting would result in cross sections of muscle fibers being cut. The specimen was mounted on a labeled cork disc with viscous embedding compound (OCT; Tissue Tek, Elkhart, IN). The mounted, labeled block was then rapidly frozen by plunging it quickly and completely into isopentane cooled with liquid nitrogen to approximately 160 °C for 30 seconds. Frozen specimens were stored at 70 °C until sectioning Limb muscles were processed in the same manner to use as controls. Sectioning of muscle was carried out in a cryostat at 19 C°. At least 5 serial, 8 tm thick transverse sections were cut. Sections were stained with Gomori trichrome and H&E to evaluate tissue orientation and general appearance of muscle fibers. Sections were stained for myosin ATPase activity with standard methods in which they were pre-incubated with alkaline solution (0.1 M sodium barbital, 0.18 M calcium chloride) at pH 10 for 15 minutes, and with acid solution (0.24M sodium 62 acetate, 0.14M sodium barbital, and 0.1M HC1) at pH 4.35 and pH 4.65 for 5 minutes. The specimens were then rinsed with wash solution (0.1 M sodium barbital, 0.18 M calcium chloride) at pH 9.4 for 3 minutes, incubated with incubating solution (0.1 M sodium barbital, 0.18 m calcium chloride, 300 mg ATP disodium salt) for 10 minutes at 37 C, and washed in 3 changes 1% calcium chloride for a total of 10 minutes. The specimens were transferred to a 2% cobalt chloride solution for 3 minutes, washed well in 6 changes of 0.01 M sodium barbital for 10 minutes, and washed in running tap water for 45 seconds. The specimens were placed in fresh 2% (NH4)2S for 35 seconds, washed well in running tap water for 90 seconds, dehydrated in ascending alcohols, cleared in xylene, and mounted in permount. The sections were examined by light microscopy for high, intermediate, and low enzyme activity judged by the intensity of intracellular staining. The muscle type was determined by matching the observed enzyme activity to the profile of muscle types of llama skeletal muscles. The serial sections were photographed with a digital camera attached to the microscope. Fiber type proportions were determined using color micrographs of serial sections. Fiber type was classified according to (Brooke and Kaiser, 1970) in which type 1 and type 2 fibers were identified by the myosin ATPase reaction after preincubation at pH 10. Type 2A, 2B, and 2C fibers were identified by the myosin ATPase reaction after preincubation at pH 4.65 and pH 4.35. 63 Ultrastructural Study Small pieces from cranial cervical, caudal cervical, middle thoracic, and distal thoracic regions of the esophagus were fixed in 2% paraformaldehyde and 3% glutaraldehyde in 0.1 M cacodylate buffer pH 7.3. The tissue was processed in tissue processor (Lynxel, Leica), postfixed in 1% 0s04 in 0.1 M cacodylate buffer, and dehydrated through graded acetone. The tissue was embedded in Epon- Araldite resin. Semithin sections (1 rim) were cut on ultramicrotome (MT-500, Sorvall) and stained with toluidine blue. Ultrathin sections (70 nm) were cut, mounted on 300 mesh hex copper grids, and stained with saturated uranyl acetate and Reynold' s lead citrate. The section was examined under an electron microscope (Zeiss 10-A TEM). Statistical Analysis All values are expressed as mean ± SD. The repeated measures ANOVA with Tukey' s Studentized Range (HSD) test for multiple comparisons of means was used. All statistical analyses were performed with SAS for Windows software program, Version 8 (SAS Institute mc, Cary, NC, USA) with a two-tailed significance criterion set atp = 0.05. 64 RESULTS Gross Anatomy The cervical esophagus lay dorsal and slightly to the left of the trachea. Once the esophagus passed through the thoracic inlet it lay dorsal to the trachea. Within the mediastinum, the thoracic esophagus crossed to the right of the aortic arch and dorsal to the heart base. In adult llamas the esophagus was approximately 120 cm long, with the cervical portion being approximately 80 cm long, and the thoracic portion approximately 40 cm long (Table 3.1). Because the first compartment (Cl) of the forestomach was in such close contact with the diaphragm the abdominal portion of the llama esophagus was very short, approximately 2 cm long. The outer diameter of the cervical portion of the esophagus was approximately 2.5 cm, enlarging some at the thoracic inlet to approximately 3 cm and then further enlarging within the thorax to 3.5 cm (Table 3.1). 65 Table 3.1 Summary of the length and outer diameter of llama esophagus from 25 llamas. Esophageal segments Length (cm) Cervical 80.00±4.49 Thoracic 41.76±3.69 Total 121.76±6.63 Esophageal regions Cranial cervical Middle cervical Thoracic inlet Middle thoracic Caudal thoracic Outer diameter (cm) p-value (among 2.54± 0.31 A 2.62±0.23 A 2.99±0.38 3.50±0.62 B C 3.89±0.77 D groups)<O.000 1 Group comparisons* * Means with the same letter are not significantly different. Histologic Anatomy The llama esophagus consisted of four layers: (1) mucosa, (2) submucosa, (3) muscularis, and (4) adventitia or serosa (Figure 3.1). The mucosal epithelium was a keratinized, stratified squamous epithelium throughout the length of the esophagus. The stratum comeum of the epithelium was composed of approximately 7-10 cell layers. Cells at the surface lacked nuclei. Lamina propria consisted of connective tissue, vascular structures, and scattered inflammatory cells. Finger-like extensions, dermal papillae, of lamina propria interdigitated with the epithelium. Muscularis 66 mucosa, identifiable only in the most distal esophagus, consisted of a few, thin, scattered strands of smooth muscle. Submucosal glands were abundant and found throughout the length of the esophagus. The glands, however, appeared to be less numerous towards the caudal end of the esophagus; the number of lobules of submucosal glands found in each region of the esophagus ranged from 42 in cross section of the cranial cervical region to 31 in the middle thoracic region (Table 3.2). The glands were ovoid or elliptical, large and small clusters or lobules of tubuloalveolar mucous glands (Figure 3.1). In each cross section throughout the esophagus, the glands were equally distributed around the wall of the esophagus. The glands abruptly disappeared in the cardia. Tunica muscularis was composed of striated muscle throughout the length of the esophagus. The thickess of tunica muscularis in thoracic segment was greater than that of the cervical segment (Table 3.2). The tunica muscularis consisted of two layers, the inner and the outer layers. Although generally the outer tunic had circularly arranged muscle fibers and the inner were longitudinal , there was also irregularity in the orientation of the muscle fibers in each one. This could be seen only by inspecting the entire cross section of the specimen. In part of the same section, the orientation was mixed. That is, in the outer tunic, the longitudinally arranged fibers alternated with the circularly arranged ones, and vice versa in the inner tunic. From the cross sections of frozen specimens, the shape of muscle fibers was smaller and less polygonal than that of limb skeletal muscle. Slight variation in muscle fiber size appeared in the distal segment of the esophagus. The blood vessels and perimysial connective tissue 67 increased in the tunica muscularis of the distal segment of the esophagus. Nerve fibers were seen between two layers of the tunica muscularis and within each layer itself, but neither the myenteric plexus nor ganglion cells were seen except in 45 5 of slides examined had 1-3 ganglion cells. In some slides the nerve fibers extending from the tunica adventitia into the tunica muscularis were seen. Figure 3.1 A typical cross section of the llama esophagus. The mucosal epithelium is keratinized stratified squamous. The submucosal glands are abundant and have ducts to transport their products to the epithelial surface. Tunica muscularis consists of two striated muscle layers separated by connective tissue bands containing small blood vessels and nerves. The adventitia is prominent. 68 Table 3.2 The thickness of tunica muscularis and the number of lobules of submucosal glands in each region of the esophagus. Cranial Cervical 3.43± 0.30 Middle cervical 3.60±0.45 Thoracic inlet 3.98± 0.47 Middle thoracic 4.39± 0.39 Group comparisons* p-value (among groups) <0.0001 Number of lobules of submucosal glands per cross section A A,B B,C C 33.6± 4.1 30.7± 5.9 Group comparisons* p-value (among groups) <0.0001 A B,C C Thickness of tunica muscularis (mm) 42.3± 3.50 35.7± 4.7 A,B * For each comparison, means with the same letter are not significantly different. Muscle Histochemical Study Type 2 fibers predominanted in both the cervical segment (99%) and the thoracic segment (70%) of the esophagus (Figure 3.2). There was a gradual increase of type 1 fibers from the cranial (1%) to the caudal segment (30%) of the esophagus. 69 A: pH 4.35 middle cervical esophagus B: pH 10 middle cervical esophagus F C: pH 4.35 caudal thoracic esophagus D: pH 10 caudal thoracic esophagus (Continued) 70 E: pH 4.35 limb muscle F: pH 10 limb muscle Figure 3.2 Myosin ATPase stained sections of the llama esophagus and limb (vastus intermedius) muscle (lOOx). Type 1 muscle fibers stained light at pH 10 and dark at pH 4.35. The reverse was true for type 2 muscle fibers. Type 2 muscle fibers were the predominant type in both cervical and thoracic portion of the esophagus. The proportion of type 1 muscle fibers was low in the cervical esophagus, but increased in the thoracic esophagus. Ultrastructural Study The longitudinally cut fibers were observed to contain A and I bands, Z lines, H bands and also distinct M lines (Figure 3.3). Muscle fiber nuclei occupied peripheral locations, several nuclei occurring in each fiber profile. Mitochondria were interspersed between the myofibrils, and tended either to lie in pairs adjacent to the Z lines, or to be disposed with their long axes parallel to the long axis of the fiber. Glycogen granules were numerous and dispersed in the I band and interfibrillar space. 71 A: Cervical esophagus B: Thoracic esophagus Figure 3.3 Esophageal striated muscle by transmission electron microscopy. Cervical esophagus (A) 16,000x. Thoracic esophagus (B) 6,300x. Typical sarcomeres consisted of Z lines, A and I bands, M lines and H zones. DISCUSSION The length of the cervical portion of the llama esophagus is approximately twice that of the thoracic portion in keeping with the long neck of the llama. This proportion is greater than that of the adult thoroughbred horse (the proportion approximately is 1.4; the total length of the esophagus is approximately 120 cm long, the cervical portion is approximately 70 cm long, the thoracic portion is approximately 50 cm long (Murray, 1998)), the cow (the proportion is approximately 1; the total esophageal length is approximately 90-95 cm long, the cervical portion is approximately 42-45 cm long, the thoracic portion is approximately 48-50 cm (Schummer et al., 1979)), and the sheep with the total esophageal length approximately 45 cm long (Getty, 1975). This data confirmed 72 the relatively longer cervical esophagus of the llama than that of the horse, cow, and sheep. The outer diameter of the llama esophagus, like the cow and sheep esophagi, significantly increases from the cranial portion to the caudal portion. However, the diameter in the llama, 2.5 cm in the cranial cervical to 3.9 cm in the caudal thoracic, is smaller than that of the cow, 3 cm in the neck to 4 cm wide and 7 cm high in the caudal thorax but bigger than that of the sheep, 1.8 cm at the pharynx to 2.5 cm at the cardia (Getty, 1975). The thickness of the tunica muscularis increases from the cranial to caudal end, and is significantly greater in the thoracic esophagus than that of the cervical esophagus. This is similar to the horse with the increase in thickness from middle cervical region, 3 mm, to 4 2 mm in the middle thoracic esophagus (Slocombe et al., 1982), but differs from the cow which is thicker in the cervical part (4-5 mm) and thinner in the thoracic part (2-3 mm) (Schummer et al., 1979). This difference may be attributed to the difference in the relative position of the neck and natural grazing and swallowing behavior. The neck in the llama lies much more upright than that of the cow. In the llama in addition to esophageal peristaltic, gravity would allow for easier passage of the bolus of food in the neck than in the cow. This is not true in the thoracic portion of the esophagus. Without gravity, the thoracic esophagus may require stronger muscle to move the bolus which is accomplished simply by increasing the muscle mass. The mucosa of llama esophagus is composed of keratinized stratified squamous epithelium. The stratum corneum of the esophageal epithelium consists of thick 73 layers of heavily keratinized cells. This character is similar to camel, cow, sheep, horse, rodents, but not human, monkey, dog, and cat (Schummer et al., 1979; DeNardi and Riddell, 1991; Fawcett and Raviola, 1994). The keratinization of the epithelium helps to prevent the injury from coarse and dry foods. The presence of muscularis mucosae in the form of a few scattered strands of smooth muscle, only in the caudal segment of the llama esophagus is similar to the camel, but it is contrary to what is found in the ruminants. In the latter, the muscularis mucosae has been reported to occur through the length of the esophagus but to be incomplete (Schummer et al., 1979). The function of muscularis mucosa in the esophagus is unclear, but in the intestine where the muscularis mucosae is well developed, its contraction increases the height of folds of mucosa. The presence of abundant submucosal glands throughout the length of the esophagus is similar to the camel (Jamdar and Ema, 1982). However, in the ruminants, horses, and cats the submucosal glands are found in the pharyngo-esophageal region only (Schummer et aT., 1979). The sumucosal glands, like minor salivary glands, secrete water and bicarbonate ion (Long and Orlando, 1999). This apparent structural modification could be explained on the basis of the habitat of the each species and food stuffs available, for an example in camels there being need for more mucous glandular secretion for the passage of food of dry an rough type through the long esophagus in a water-scarce environment which is also true of much of the forage of the Andean altiplano where the llama originated. The secretions of these glands may 74 also contribute to the increased buffering capacity of Cl of the llama compared to the ruminants. Tunica muscularis in most species can be divided into two layers: the inner and the outer. Tunica muscularis varies greatly among species with respect to the proportion of the striated and smooth muscles. In the human, the proximal five percent of esophageal length including upper esophageal sphincter is striated, 35 to 40 percent is mixed with an increasing proportion of smooth muscle distally, and the distal 50 to 60 percent is entirely smooth muscle (Meyer et al., 1986). In the cat and horse, the cranial two thirds of the esophagus consists of striated muscle and the caudal third of smooth muscle; in the pig, the esophagus consists of striated muscle except the short segment near the cardia with smooth muscle; in the dog, rodent, ruminant, and camel, the tunica muscularis consists entirely of striated muscle (Goetsch, 1910; Schummer et aL, 1979; Jamdar and Ema, 1982). The tunica muscularis in llamas, like ruminants and camels, consists of two layers of striated muscle throughout the entire length of the esophagus. The orientation of muscle fibers also varies among species. In the human and monkey with smooth muscle predominant in the esophagus, the orientation of muscle fibers is similar to the other regions of gastrointestinal tract in which the inner layer is circular and outer one is longitudinal (Whitmore, 1982; Brown et al, 1978). In the horse, the striated muscle layers are oriented obliquely to one another; the muscular layers become oriented in more of an inner circular and outer longitudinal configuration in the caudal esophagus (Schummer et al., 1979). In the dog, ruminant, and camel, the 76 composed of differently shaped ganglia and interconnecting nerve fiber strands. From the cervical toward the thoracic segment of the esophagus the density and size of myenteric ganglia increase and nerve fiber strands exhibit thicker diameters (Teixeira et al., 2001; Vittoria, 2000). The myenteric plexus in the llama is similar to that in the camel in which it is scanty and difficult to locate (Jamdar and Ema, 1982). The function of the myenteric plexus in striated muscle is still unclear, but recent studies have showed co-innervation between the nerve fibers from the vagus and the plexus on the endplates of striated muscle in the guinea-pig and rat esophagus (Zhou et al., 1995; Neuhuber et al., 2001). The muscle fiber types in llama esophagus are similar to that in ruminants in which, in the cranial part of the esophagus, the muscle fiber type is type 2 predominantly (99% in llamas, cow and sheep), but the percentage of type 1 fibers increases in the thoracic segment (30% in llamas, 30 % in sheep, and 36% in cows) (Mascarello et al., 1984). In the ruminants and donkey esophagi, type 2A and 2 C are found and classified with immunohistochemical characteristics identical to those of the same fiber types found in control skeletal muscle (Mascarello et al., 1984). In the carnivores, the esophageal striated muscle is composed of a very small percentage of type 1 and 2 C fibers, but the predominant type, called type 2oes, is very different histochemically and immunohistochemically from all the fiber types (1, 2A, 2B, and 2C) present in the control muscle (Mascarello et al., 1984). However, type 2 is also the predominant type in the esophagus of the rat, guinea-pig, and rabbit whose esophagi consist entirely of striated muscle (Gruber, 77 1978; Whitmore, 1978; O'Brien, 1982). Fiber types may reflect the difference of the physiological function between the two segments of the esophagus. From the esophageal manometric study in the llama (Chapter 2), a burp usually occurs after Cl contraction. Then, secondary peristalsis in the thoracic segment of the esophagus usually occurs after the burp. Secondary peristalsis rarely occurs in the cervical esophagus of normal llamas. When a first compartment (Cl) contraction occurs, in addition to gas, small amounts of the fluid content in Cl may enter into the thoracic portion of the esophagus. The thoracic esophagus uses the secondary peristalsis mechanism to clear the fluid out of the esophagus. This occurs rarely in the cervical esophagus because it is far away from Cl and it lies vertically so that the gravity could help the cervical esophagus clean without secondary peristalsis. Cl contraction and burp cycles would occur the entire day. Thus secondary peristalsis in thoracic esophagus also occurs all day long. The thoracic esophagus would need the type 1 of muscle fiber, which is the less fatigable type, to perform this task. Generally, ultrastucture of esophageal striated muscle in mice, guinea pigs, and marmosets is similar to that of skeletal muscle (Samarasinghe, 1972; Whitmore, 1983). However, There are some differences, for instance, motor endplates of esophageal striated muscle fibers in guinea pigs are more simple than those of the skeletal muscle fibers (Whitmore, 1983), and co-innervation of cholinergic motoneurons and enteric neurons on motor endplates of esophageal striated muscle fibers was observed in guinea pigs and rats (Zhou et al., 1996; Kuramoto et al., 78 1996; Won et al., 1998; Neuhuber et al., 2001). Ultrastructure of the esophageal striated muscle in the llama is also similar to the skeletal muscle in which it consists of typical A , I, and H bands and Z and M lines. Unfortunately, neuromuscular junctions were not observed in this study. Anatomically, the esophagus of the llama is more similar to that of the camel than that of the cow and sheep. This is expected as llamas and camels are both camelidae and phylogenitically different from the true ruminants. The length of cervical portion is almost twice that of the thoracic portion in keeping with the long neck. The diameter in thoracic portion is greater than that of the cervical portion. The submucosal glands are abundant throughout the esophageal length. The tunica muscularis consists of two layers of striated muscles fibers throughout the whole length. The arrangement of muscle fibers in the two layers is mixed. Small nerve fibers are present between the two layers. Muscle fiber type distribution is similar to that of the ruminant with type 2 muscle fibers predominating throughout the esophageal length. The proportion of type 1 muscle fibers gradually increases from cranial to the caudal portion of the esophagus which may be explained by the need for more frequent secondary peristalsis in the caudal esophagus. Ultrastructure of the esophageal striated muscle is similar to that of general skeletal muscle. This study provides the first detailed anatomy description for the esophagus of the llama to be used as a basis for further studies of esophageal malfunction. 79 REFERENCES Asaad K, Rahman SA, Nawar NNY et al. Intrinsic innervation of the oesophagus in dogs with special reference to the presence of muscle spindles. Acta Anat 1983;1 15:91-96. Brooke MH, Kaiser KK. Muscle fiber type: how many and what kind? Arch Neurol 1970;23 :369-379. Brown FC, Gideon RM, Voelker FA, et al. Muscle function and structure of the esophagus of the baboon (Papio anubis). Am J Vet Res 1978;39: 1209-1211. Christensen J, Rick GA, Robison BA, et al. Arrangement of the myenteric plexus throughout the gastrointestinal tract of the opossum. Gastroentero! 1983 ;85 :890899. Christensen J, Robison BA. Anatomy of the myenteric plexus of the opossum esophagus. Gastroenterol 1982;83:1033-1042. Clifford DH, Gyorkey F. Myenteric ganglia! cells in dogs with and without achalasia of the esophagus. J Am Vet Med Assoc 1967;! 50;205-2 11. Dellmann DT, Brown EM. Digestive system. In: Textbook of Veterinary Histology. Philadelphia: Lea and Febiger, 1976;225-226. DeNardi FG, Riddell RH. The normal esophagus. Am J Surg Pathol 199 1;15:296309. Fawcett DW, Raviola E. The esophagus and stomach. In: Fawcett, ed. A Textbook of Histology, 12th ed. New York: Chapman & Hall, 1994;593-616. Fowler ME. Digestive system. In: Medicine and Surgery of South American Camelids: Llama, Alpaca, Vicuna, Guanaco. 2' ed. Iowa: Iowa State University Press, 1998;305-359. Getty R. Digestive system of the sheep. In: Sisson and Grossman's The Anatomy of the Domestic Animals, 5t1 ed. Philadelphia: WB Saunders Co, 1975;477-484. Goetsch E. The structure of the mammalian esophagus. Am J Anat 1910;10:!-40. Gruber H. Motor innervation of the striated oesophagus muscle. J Neurol Sci 1978 ;36:4 1-53. 80 Jacobowitz D, Nemir P. The autonomic innervation of the esophagus of the dog. J Thorac and Cardiovasc Surg 1969;58:678-684. Jamdar MN, Ema AN. The submucosal glands and the orientation of the musculature in the oesophagus of the camel. J Anat 1982;135:165-171. Kuramoto H, Kato Y, Sakamoto H, et al. Galanin-containing nerve terminals that are involved in a dual innervation of the striated muscles of the rat esophagus. Brain Res 1996;734:186-192. Long JD, Orlando RC. Esophageal submucosal glands: structure and function. Am J Gastroenterol 1999;94:2818-2824. Mascarello F, Rowlerson A, Scapolo PA. The fibre type composition of the striated muscle of the esophagus in ruminants and carnivores. Histochemistry 1984;80: 277-288. Meyer GW, Austin RM, Brady CE, et aT. Muscle anatomy of the human esophagus. J Clin Gastroenterol 1986;8:131-134. Neuhuber WL, Eichhorn U, Worl J. Enteric co-innervation of striated muscle fibers in the esophagus: just a "hangover"? Anat Rec 2001 ;262(1):41-46. O'Brien GM. Histochemistry of the muscularis externa of the oesophagus in rabbit. Proc Austr Physiol Pharm Soc 1982;13:23P. Samarasinghe DD. Some observations on the innervation of the striated muscle in the mouse oesophagus - an electron microscope study. J Anat 1972; 112:173-184. Schummer A, Nickel R, Sack WO. The alimentary canal. In: The Viscera of Domestic Mammals. New York: Springer-Verlag, 1979;99-202. Slocombe RF, Todhunter RJ, Stick JA. Quantitative ultrastructural anatomy of esophagus in different regions in the horse; effects of alternate methods of tissue processing. Am J Vet Res 1982;43: 1137-1142. Teixeira AF, Vives P, Krammer HJ, et al. Structural organization of the enteric nervous system in the cattle esophagus revealed by wholemount immunohistochemistry. Ital J Anat Embryo! 2001;106(2) Suppl 1:313-321. Vittoria A, Costagliola A, Carrese E, et al. Nitric oxide-containing neurons in the bovine gut, with special reference to their relationship with VIP and galanin. Arch Histol Cytol 2000;63:357-368. 81 Whitmore I. Histochemical fibre types in striated muscle from the guinea pig esophagus. Experientia 1978 ;34: 1632. Whitmore I. Oesophageal striated muscle arrangement and histochemical fibre types in guinea-pig, marmoset, macaque and man. J Anat 1982;134:685-695. Whitmore I. The ultrastructure of oesophageal striated muscle in the guinea-pig and marmoset. Cell Tissue Res 1983;234:365-376. Won J, Fischer J, Neuhuber WL. Nonvagal origin of galanin-containing nerve terminals innervating striated muscle fibers of the rat esophagus. Cell Tissue Res 1998 ;292:453-46 1. Zhou DS, Desaki J, Komuro T. Neuromuscular junctions of longitudinal and circular muscle fibers of the guinea-pig esophagus and their relation to myentenic plexus. J Auton Nerv Syst 1996;58:63-68. 82 CHAPTER 4. CASE REPORT: TWO LLAMAS WITH MEGAESOPHAGUS AND ONE LLAMA WITH ESOPHAGEAL MOTOR ABNORMALITY ABSTRACT Two llamas with megaesophagus (a nine-year-old, 102.27 kg, male, and a fiveyear-old, 98.18 kg, female) and a nine-year-old, 213.63 kg, female llama with esophageal motor abnormality were evaluated by solid-state esophageal manometry. Resting pressure measurements, water swallows, and balloon distention techniques were applied to all animals. Peristaltic parameters of the esophageal peristalsis induced by a water swallow were analyzed. Then, all three animals were humanely killed by an intravenous overdose of pentobarbital at 0.45 mllkg body weight. The length and diameter of the esophagus were measured. Samples for microscopic studies were taken at the levels of cranial cervical, middle cervical, thoracic inlet, middle thoracic, and caudal thoracic esophagus. Esophageal peristalsis in llamas with megaesophagus was abnormal. Although the proximal portion of the esophagus still functioned, the amplitude of contraction gradually decreased and more than half of the distal esophagus was aperistaltic. Peristalsis in the llama with esophageal abnormality could be detected throughout the esophagus, but the amplitude of contraction was significantly lower than that of normal llamas and incomplete peristalsis was also observed. In addition, an extra high pressure zone, approximately 2 cm long, was detected in the thoracic inlet region of the esophagus. In all three animals, the percentage of swallows that were 83 propagated into the esophageal body was decreased by 5% from the normal (normal = 100%, all three animals = 95%). In llamas with megaesophagus, atrophy and hypertrophy of muscle fibers were found at the middle cervical, thoracic inlet, middle thoracic, and caudal thoracic regions of the esophagus. Muscle fibers in the cranial cervical esophagus of llamas with megaesophagus and in all regions of the llama with esophageal motor abnormality were similar to that of normal llamas. The distribution of the lesions leads us to believe that innervation of the proximal cervical esophagus differs from the remainder of the esophagus. The third llama had physiologic deficits, but not demonstrable changes in muscle. Possibly she was an earlier stage of the disease. INTRODUCTION The most common esophageal problem in llamas is megaesophagus, a term referring to generalized esophageal dilation. Overall incidence is difficult to determine. In an informal survey of veterinarians in the Northwest who routinely treat SAC's it was found that most were aware of at least one SAC with megaesophagus in their practice area. In a retrospective study of 15 llamas with acquired megaesophagus animals ranged in age of onset from 13 months to 9.5 years. Etiology was not determined in 11 animals. Megaesophagus in one llama could be attributed to organophosphate toxicity, vagal neuropathy in one and degenerative myopathy in two llamas (Watrous et al., 1995). Megaesophagus in a nine year old, male llama was also reported with persistent right aortic arch (Butt et 84 al., 2001). In this study two llamas with megaesophagus and one with esophageal motor problems were evaluated by the physiological and anatomic methodologies previously developed in ten normal llamas (Chapter 2, 3). CASE REPORTS Case 1 A nine-year-old, 102.27 kg, male llama presented with chronic weight loss, and bouncing up and down (associated with swallowing) of the bolus of food in the neck. The skin around the mouth and nose, and the walls of the stall in the animal hospital were stained with the green color of the bolus contents bouncing from compartment 1 (Cl) of the llama stomach. He also had a history of episodes of choking that was becoming more difficult to relieve. Radiographs demonstrated a greatly dilated distal cervical and thoracic esophagus with barium retention in the thoracic esophagus. Case 2 A five-year-old, 98.18 kg, female llama presented with chronic weight loss, prominent periodic abdominal contractions associated with bouncing up of the bolus of the food into the neck. The animal responded to the bouncing-up bolus by swallowing. Radiographic findings were similar to case 1. 85 Case 3 A nine-year-old, 213.63 kg, female llama presented with no clinical signs of esophageal disease and originally was to be used as a control animal for the llama esophageal study. She was evaluated by esophageal manometry twice at a six month intervals. The results were obviously different from the normal control llamas and from the time of the first evaluation on her. On radiographic evaluation there was no obvious dilation of the esophagus but there was some food in the thoracic esophagus. Barium was retained in the thoracic esophagus after a barium swallow. Esophageal Manometric Examination Each case was evaluated with esophageal manometry. Esophageal manometric equipment, procedures, and data analyses were similar to that used with the normal llama (Chapter 2). Briefly, solid-state esophageal manometry was used to measure resting baseline pressures and the esophageal response to water swallows, as well as to balloon distention. Case 3 was evaluated twice at a six month interval. Gross and Histologic Examination All cases were humanely killed by an intravenous overdose of pentobarbital at 0.45 ml/kg body weight. The length of the cervical and thoracic portion of the 86 esophagus was measured. The diameter and thickness of the muscularis was measured at the cranial cervical, middle cervical, thoracic inlet, middle thoracic, and caudal thoracic esophagus. Tissue samples were taken from each level throughout the esophagus as well as multiple levels of the vagus nerve. All tissues were fixed in 10% formalin and embedded in paraffin. The sections were stained with hematoxylin and eosin (H&E) and examined with a light microscope. Muscle Histochemical Examination Fresh samples from five regions (cranial cervical, middle cervical, thoracic inlet, middle thoracic, and caudal thoracic) of the esophagus were frozen in isopentane cooled with liquid nitrogen, transversally cut at 8 p.m with a cryostat microtome, and stained with H&E, Gomori trichrome, and myosin ATPase at pH 10, 4.65, and 4.35 using standard techniques. Electron Microscopic Examination Fresh samples of the esophagus and vagus nerve from four regions (cranial cervical, caudal cervical, middle thoracic, and caudal thoracic) of the esophagus were fixed in 2% paraformaldehyde and 3% glutaraldehyde in 0.1 M cacodylate buffer pH 7.3, post-fixed inl% 0s04 in 0.1 M cacodylate buffer, dehydrated in acetone, and embedded Epon-Araldite resin. Semithin sections (1 /Lm) were cut on ultramicrotome (MT-500, Sorvall) and stained with toluidine blue. Ultrathin sections (70 nm) were cut, mounted on 300 mesh hex copper grids, and stained 87 with saturated uranyl acetate and Reynold's lead citrate. The sections were examined with a transmission electron microscope (Zeiss 10-A TEM). Statistical Analysis Student's t-test was used to compare diameter at each region of the esophagus in normal llamas and all three cases. This method was also applied to thickness of tunica muscularis. RESULTS Esophageal Manometric Findings In case 1 and 2 (megaesophagus cases), the normal resting high pressure zone of the lower esophageal sphincter could not be detected. In case 1 and 2, resting pressures periodically increased in association with Cl contraction in the thoracic esophagus and coincided with the bouncing up and down of the bolus observed in the neck in the cervical esophagus (Figure 4.1). Resting pressures of the upper esophageal sphincter were approximately 7 mrnHg in case 1 and 9 mmHg in case 2. Resting pressures in case 3 were similar to those of normal llamas in which resting pressures of the lower and upper esophageal sphincters were approximately 8 and 15 mm}Ig respectively. However, there was an extra high pressure zone in the thoracic inlet region of the esophagus (110 to 113 cm from the nares) (Figure 4.2) with a pressure of 25 mmHg. 88 A B Wt,,tAIVfttttPftdt.1P C'r\flU ?,. Figure 4.1 Resting pressures in the cervical (A) and thoracic (B) esophagus of case 1. The fluctuations of baseline pressures (arrows) were associated with Cl contractions and bouncing up and down of the bolus in the cervical esophagus. 89 tCTPO$C5. Tracing 3 Figure 4.2 Station pull-through technique shown the abnormal resting pressure of the esophageal body in case 3. Tracing 1 demonstrated the high pressure zone (arrow) in the thoracic inlet region of the esophagus. 90 After a water swallow, primary peristalsis was initiated and the peristaltic wave propagated down the esophagus. However, there were approximately 5% of swallows that failed to initiate peristalsis or did not propagate into the proximal esophagus from all three animals (Figure 4.3). In both megaesophagus llamas, the amplitude of the peristaltic wave gradually decreased and the wave disappeared below the middle cervical esophagus, at approximately 35% of esophageal length; no peristalsis or esophageal contraction was detected by esophageal manometry below this region (Figure 4.4). 91 Swallow marker Tracing I Tracing 2 Tracing 3 Tracing 4 Figure 4.3 Failure of a swallow to initiate esophageal peristalsis. A swallow marker (arrow) was put in the pharynx. The first proximal tracing was at 45 cm from the flares. No peristalsis was observed in the esophagus (tracing 1,2, 3 and 4). 92 Swallow marker 60cm 70cm 80cm 90cm Figure 4.4 Primary esophageal peristalsis in a llama with megaesophagus. Note that esophageal peristalsis was terminated between 70 and 80 cm (distance from the nares), with approximately 65% of esophageal length being aperistalsis. 93 The amplitudes of contraction in the cervical esophagus were approximately 54 mmHg in case 1 and 21 mrnHg in case 2 (Figure 4.5). This was significantly lower than that of normal llamas in the same region (approximately 78 mmHg). The duration of contraction was 0.33 second in case 1 and 0.34 second, similar to normal llamas (0.40 second). Peristaltic velocity was 47.3 cm/s in case 1 and 35.5 cm/s in case 2; this was significantly higher and lower than that of normal llamas (41.9 cmls). In case 3, the amplitude of contraction at the time of the second measurement (29±15 mmHg in cervical and 28±14 mmHg in thoracic portions), done six months later after the first one, was significantly lower than that of the first one (60±25 mmHg in cervical and 61±27 mmHg in thoracic portions) in both cervical and thoracic portions (Figure 4.5). In case 3, incomplete peristalsis frequently occurred in both cervical and thoracic portions (Figure 4.6). The esophagus of both megaesophagus cases (case 1 and 2) still had ability to pull the balloon down during balloon distention but required a balloon volume of at least twice the volume used in normal llamas. The propulsive force generated from balloon distention was very weak. In case 3, the same balloon volume used in normal llamas had the ability to stimulate the esophagus to generate propulsive force, but it was weaker than that of normal llamas. 94 A normal llama Megaesophagus(case 1) -9 1 11 21 31 41 51 61 71 81 91 Percent of esophageal length Figure 4.5 Plots of means of the amplitude of contraction from a normal llama and all three cases. Esophageal peristalsis terminated at approximately 35% of esophageal length in llamas with megaesophagus (case 1 and 2). In case 3, amplitude of contraction was significantly lower when compared to a normal llama. 95 I ;flDm!iEuhiuniwIqp !?1J c,nt.sIIIH9ttildIUItltUhlI4II I I ¶ 4ffiH unumrnr in'' Jas$ilr_mzraJlftjj HI USIWI4 r,,,,,-., FyIbMIIrti i*Q) H Ii III :19flt1!W4!j? iii ll fthl1IHø .ini irnuuii fiffinihi(ffifi]t ! :blfl MilIIflI III1IIfl hffiHft1HMUlIihiinll _________________ qwujmu 91 1g1Ej ijk I'll! l"Ith'HIkffil flu Iufl iInp;t4 H fthlIDll DII IUL Ull II r ii hUll 011th PiIuilliIlliliII Figure 4.6 Incomplete peristalsis in case 3. Tracing 1 was a swallow marker. Peristaltic contraction was weak in tracing 2 and 3, and was not detected in tracing 4 and 5. 96 Gross and Histologic Findings Lengths of the esophagus were (135 cm; 90, 45 cm case 1), (123 cm; 82, 41 cm case 2) and (135 cm; 89, 46 cm case 3). The diameter of the esophagus ranged from 2.5 cm in the cranial cervical portion to 8.5 cm in the distal thoracic esophagus (Table 4.1). The overall thickness of the tunica muscularis was significantly increased in the cranial cervical region of affected animals, and was significantly decreased in samples from the thoracic inlet and middle thoracic regions. Table 4.1 Diameter of esophagus and thickness of tunica muscularis in various regions throughout the length. Cranial cervical Middle cervical Thoracic inlet Middle thoracic Caudal thoracic Diameter (cm) Normal All three llamas cases 2.54±0.31 2.83±0.29 0.62 Thickness (mm) All three Normal llamas cases 3.43±0.30 3.87±0.12 p-value pvalue 0.04 2.62±0.23 2.87±0.3 1 0.21 3.60±0.45 3.53±0.25 0.82 2.99±0.38 4.23±1.07 0.002 3.98±0.47 3.40±0.36 0.07 3.50±0.62 5.40±0.70 <0.0001 4.39±0.39 3.43±0.49 0.04 3.89±0.77 6.90±1.39 <0.0001 NE NE NE NE = Not Examined 97 Routine H&E stained sections revealed atrophy and hypertrophy of muscle fibers of sample sections from middle cervical, thoracic inlet, middle thoracic, and caudal thoracic esophagus of llamas with megaesophagus. In examination of vagal nerve there was no evidence of vagal neuropathy in all three cases. There was also no evidence of neuropathy of intramuscular nerve of esophageal striated muscle from affected animals. Muscle Histochemical Findings In both megaesophagus cases (case 1 and 2), esophageal striated muscle of the cranial cervical region was similar to that of normal llamas in terms of fiber type composition, in which type 2 was predominant (approximately 99%) and morphology. Numerous small groups of severely angular atrophied muscle fibers were observed in the sample sections of both megaesophagus cases (case 1 and 2) at middle cervical, thoracic inlet, middle thoracic, and caudal thoracic regions of the esophagus (Figure 4.7). The atrophic fibers were both type 1 and type 2, but atrophy of type 2 fibers predominated, in keeping with the type 2 predominance of the muscle (Figure 4.7). Hypertrophy ofboth type 1 and 2 muscle fibers was also seen in each slide that had atrophic muscle fibers (Figure 4.7). In case 3, the muscle fiber types and morphology were similar to that of normal llamas. 98 A. Cranial cervical esophagus C. Middle thoracic esophagus B. Cranial cervical esophagus D. Middle thoracic esophagus (Continued) 99 E. Limb muscle F. Limb muscle Figure 4.7 Myosin ATPase stained sections at pH 10 of esophageal striated muscle and limb (vastus intermedius) muscle from the normal llamas (left side) and llamas with megaesophagus (right side) (lOOx). Type 1 muscle fibers were stained light and type 2 muscle fibers were stained dark. Type 1 muscle fibers were not demonstrated in the cranial cervical esophagus (A and B) because the proportion of type 1 fibers was very small. There was no difference between the normal llamas and llamas with megaesophagus in the cranial cervical esophagus. In the middle thoracic esophagus, small group atrophy and hypertrophy of type 1 and type 2 muscle fibers were observed in the llama with megaesophagus (D). Limb muscle (E and F) was not different between the normal llama and a llama with megaesophagus. Electron Microscopic Findings Myofibrillar loss and disorganization were observed in the esophageal striated muscle of thoracic esophagus of llamas with megaesophagus (Figure 4.8). No evidence of vagal neuropathy was elucidated in affected animals. 100 A. A normal llama B. A llama with megaesophagus Figure 4.8 Esophageal striated muscle by transmission electron microscopy. Thoracic esophagus from a normal llama (A) 6,300x, and from a llama with megaesophagus (B) 3,150x. Note that myofibnllar loss and disorganization were observed in the esophageal striated of a llama with megaesophagus. DISCUSSION Megaesophagus, a descriptive term for esophageal dilation, may be a manifestation of a wide variety of diseases including neuromuscular, obstructive, toxicological diseases and miscellaneous disorders - these have been reviewed by Guilford (1990) and Watrous (1995). Thus, the cause of megaesophagus in an individual case may be difficult to determine. However, a defect in any part of the neuromuscular pathway that controls esophageal peristalsis can result in esophageal motor dysfunction. This pathway consists of sensory receptors in the esophagus, afferent nerve fibers in the vagus and its branches, the brain stem and CNS where the neurons responsible for esophageal peristalsis are located, efferent nerve fibers, 101 the neuromuscular junction, and the esophageal striated musculature itself. Any disruption of esophageal muscle or the central, afferent, or efferent pathways that control esophageal motility could theoretically result in megaesophagus. For instance, myositis and myopathy associated with a variety of diseases, including systemic lupus erythematosis (Boudrieu and Rogers, 1985; Krum et al., 1977), trypanosomiasis (Marsden and Hagstrom, 1968; Berger et al., 1991), glycogen storage disease (Walvort et al., 1984), polymyositis (Boudrieu and Rogers, 1985; Komegay et al., 1980), and advanced dermatomyositis (Haupt et al., 1985) have produced sufficient esophageal muscle damage to cause megaesophagus in dogs. Neuromuscular junction disease in botulism (Darke et al., 1976; Van Nes, 1986), congenital or acquired myasthenia gravis (Boudrieu and Rogers, 1985; Mason, 1976; Miller et al., 1983) and chronic anticholinesterase administration (Harris et al., 1960) have been shown to produce esophageal dilation in dogs and cats. Megaesophagus due to myasthenia gravis is a relatively common cause of secondary megaesophagus especially in the dog (Shelton et al., 1990; Shelton et a!, 1997). Various types of peripheral neuropathy and motor neuronopathy, including polyradiculoneuritis (Boudrieu and Rogers, 1985), ganglioradiculitis (Cummings et al., 1983), demyelinating neuropathies such as thallium toxicosis (Zook and Gilmore, 1967), axonopathies such as canine giant axonal neuropathy (Duncan and Griffiths, 1981), and spinal muscular atrophy (Shell et al., 1987) have resulted in megaesophagus. 102 In a retrospective study of 15 llamas with acquired megaesophagus, age of onset ranged from 13 months to 9.5 years. Etiology was not determined in 11 animals. Megaesophagus in one llama could be attributed to organophosphate toxicity, vagal neuropathy in one and degenerative myopathy in two llamas (Watrous et al., 1995). Megaesophagus in a nine year old, male llama was also reported with persistent right aortic arch (Butt et al., 2001). Megaesophagus has been reported more frequently in dogs than other species (Shelton et al., 1997). There are three distinct clinical entities associated with canine megaesophagus; congenital megaesophagus, acquired idiopathic megaesophagus, and secondary megaesophagus. In the study of 74 cases of megaesophagus in dogs due to hypomotility, 9 % were congenital, 74% idiopathic, and 17% secondary to a specific neuromuscular dysfunction (Twedt, 1995). Peripheral neuropathies, laryngeal paralysis, acquired myasthenia gravis, esophagitis, chronic or recurrent gastric dilation, and old age were associated with an increased risk of developing megaesophagus in dogs (Gaynor et al., 1997). Myasthenia gravis (MG) is the most common cause of secondary megaesophagus in dogs (Yam et al., 1996). Megaesophagus has been reported in the other species such as horses (Bowman et al., 1978; Murray et al., 1988;), cows (Anderson et al., 1984; Vestweber et al., 1985; Ross et al., 1986; Bargai et al., 1991), cats (Hoenig et al., 1990, Maddison and Allan, 1990), domestic ferret (Harms and Andrews, 1993), mice (Randelia and Lalitha, 1988), and a goat (Parish et al., 1996). In this report, it was clear that proximal esophageal portion of llamas with megaesophagus was still functioning but more than half of the distal esophagus was 103 aperistaltic. Thickening of the tunica muscularis would indicate a compensatory hypertrophy in that region. In both megaesophagus cases (case 1 and 2), manometric findings related to anatomic findings in that the diameter and the histologic findings of the cranial cervical portion of the esophagus are normal. Small group and large group atrophy of type 1 and type 2 muscle fibers, and mixed with markedly hypertrophic type 1 and type 2 muscle fibers were found in sections of middle cervical esophagus and caudal thoracic esophagus. The major causes of muscle fiber atrophy are denervation, disuse, malnutrition, cachexia, and senility (McGavin and Valentine, 2000). In this study, the findings in the middle cervical and thoracic esophageal striated muscle were consistent with denervation atrophy. This finding is similar to case 1 in the previous study (Watrous et al., 1995) that was examined with electromyography. The electromyographic abnormality score based on frequency of fibrillation potentials, positive sharp waves, and abnormal high frequency discharges was normal in the proximal portion of the esophagus but gradually increased in the distal portion. These abnormalities (fibrillation potentials, positive-sharp waves, and abnormal high frequency discharges) are signs of denervation. In addition, the percentage of swallows that were propagated into the body of the esophagus in llamas with megaesophagus was decreased to approximately 95% (normal value 100%). From the esophageal manometric study in dogs that were treated with acrylamide and that developed peripheral neuropathy and megaesophagus, the percentage of swallows that initiated peristalsis or 104 propagated into the body of the esophagus after pharyngeal contraction declined as animals developed acrylamide neuropathy (Satchel!, 1990). The distribution of the lesions leads us to believe that innervation of the proximal cervical esophagus differs from the remainder of the esophagus. Two llamas with megaesophagus in this report had both physiological and anatomical abnormalities throughout the esophagus except in the cranial cervical esophagus. The physiological findings correlated with the anatomical findings in that muscle fibers of the aperistaltic segment demonstrated atrophy and hypertrophy consistent with denervation atrophy. The third llama had physiologic deficits, but not demonstrable changes in muscle. Most likely she was an earlier stage of the disease. REFERENCES Anderson NV, Vestweber JG. Hiatal hernia and segmental megaesophagus in a cow. JAm Vet Med Assoc 1984;184:193-195. Bargai U, Nanthan AT, Pearl S. Acquired megaesophagus in a heifer. Vet Radiol 1991 ;32:259-260. Berger SL, Palmer RH, Hodges CC, et al. Neurologic manifestations of trypanosomiasis in a dog. J Am Vet Med Assoc 1991;198:132-134. Bowman KF, Vaughan JT, Quick CB, et al. Megaesophagus in a colt. J Am Vet Med Assoc 1978;172:334-337. Boudrieu RJ, Rogers WA. Megaesophagus in the dog: A review of 50 cases. J Am Vet Med Assoc 1985;21:33-40. Butt TD, Macdonald DG, Crawford WH. Persistent right aortic arch in a mature llama. VetRec 2001;148:118-119. 105 Cummings JF, DeLahunta A, Mitchell WJ. Ganglioradiculitis in the dog. Acta Neuropathol 1983 ;60:29-39. Darke PGG, Roberts TA, Smart JL, et al. Suspected botulism in foxhounds. Vet Rec 1976;99:98-100. Duncan ID, Griffiths JR. Canine giant axonal neuropathy; some aspects of its clinical, pathological and comparative features. J Small Anim Pract 1981;22:491501. Gaynor AR, Shofer FS, Washabau RJ. Risk factors for acquired megaesophagus in dogs. JAm Vet Med Assoc 1997;211:1406-1412. Harms CA, Andrews GA. Megaesophagus in domestic ferret. Lab Anim Sci 1993;43:506-508. Harris LD, Ashworth WD, Ingelfinger FJ. Esophageal aperistalsis and achalasia produced in dogs by prolonged cholinesterase inhibition. J Clin Invest 1960;39: 1744-1751. Haupt KB, Prieur DJ, Moore MP, et al. Familial canine dermatomyositis: Clinical electrodiagnosis, and genetic studies. Am J Vet Res 1985;46:1861-1869. Hoenig M, Mahaffey MB, Parnel GF, et al. Megaesophagus in two cats. J Am Vet Med Assoc 1990;196:763-765. Kornegay JN, Gorgacz EJ, Dawe DL, et al. Polymyositis in dogs. J Am Vet Med Assoc 1980;176:431-438. Krum SH, Cardinet GH, Anderson BC, et al. Polymyositis and polyarthritis associated with systemic lupus erythematosis in a dog. J Am Vet Med Assoc 1977;170:61-64. Maddison JE, Allan GS. Megaesophagus attributable to lead toxicosis in a cat. J Am Vet Med Assoc 1990;197:1357-1358. Marsden PD, Hagstrom JWC. Experimental Trypanosoma cruzi infection in beagle puppies. Trans R Soc Trop Med Hyg 1968;62:816-824. Mason KV. A case of myasthenia gravis in a cat. J Small Anim Pract 1976;17:467472. 106 McGavin MD, Valentine BA. Muscle. In: McGavin MID, Canton W, Zachary, JF, eds. Thomson's Special Veterinary Pathology. St. Louis: Mosby mc, 2000;461498. Miller LM, Lennon VA, Lambert EH, et al. Congenital myasthenia gravis in 13 smooth Fox Terriers. J Am Vet Med Assoc 1983;182:694-702. Murray MJ, Ball MM, Parker GA. Megaesophagus and aspiration pneumonia secondary to gastric ulceration in a foal. J Am Vet Med Assoc 1988;192:381-383. Parish SM, Middleton JR, Baldwin TJ. Clinical megaesophagus in a goat with thymoma. VetRec 1996;139:94. Randelia HP, Lalitha VS. Megaesophagus in ICRC mice. Lab Anim 1988;22:23-26. Ross CE, Rebhun WC. Megaesophagus in a cow. J Am Vet Med Assoc 1986; 188:623-624. Satchell PM. The neuropathic oesophagus. A radiographic and manometric study on the evolution of megaesophagus in dogs with developing axonal neurophathy. Res Vet Sci 1990;48:249-255. Shell LG, Jortner BS, Leib MS. Spinal muscular atrophy in two Rottweiler littermates. J Am Vet Med Assoc 1987;190:878-880. Shelton GD, Schule A, Kass PH. Risk factors for acquired myasthenia gravis in dogs: 1,154 cases (1991-1995). JAm Vet Med Assoc 1997;211:1428-1431. Shelton GD, Willard MID, Cardinet GH, et al. Acquired myasthenia gravis: selective involvement of esophageal, pharyngeal, and facial muscle. J Vet Intern Med 1990; 4: 28 1-284. Twedt DC. Disease of the esophagus. In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 4t ed. Philadelphia: WB Saunders Co, 1995;! 124-1142. Van Nes JJ. Electrophysiological evidence of peripheral nerve dysfunction in six dogs with botulism type C. Res Vet Sci 1986;40:372-376. Vestweber JG, Leipold HW, Knighton RG. Idiopathic megaesophagus in a calf: clinical and pathologic features. J Am Vet Med Assoc 1985;187:1369-1370. 107 Walvort HC, VanNess JJ, Stokhof AA, et al. Canine glycogen storage disease type II: A clinical study of four affected Lapland dogs. J Am Anim Hosp Assoc 1984;20:279-286. Watrous BJ, Pearson EG, Smith BB, et al. Megaesophagus in 15 llamas: a retrospective study. J Vet Intern Med 1995 ;9:92-99. Yam PS, Shelton GD, Simpson SW. Megaesophagus secondary to acquired myasthenia gravis. J Small Anim Pract 1996;37:179-183. Zook BC, Gilmore CE. Thallium poisoning in dogs. J Am Vet Med Assoc 1967;151:206-217. 108 CHAPTER 5. GENERAL CONCLUSION The main functions of the llama esophagus are to propel a food bolus from the pharynx to the first compartment (Cl) of the llama stomach and to transport the regurgitated bolus back into the mouth for rechewing and swallowing. These tasks are accomplished by means of esophageal peristalsis and reverse peristaltic contractions. Only primary esophageal peristalsis in response to a water swallow and secondary esophageal peristalsis in response to balloon distention was studied. Esophageal peristaltic parameters were analyzed. Amplitude of contraction and pressure change per unit time are the greatest in the pharygoesophageal region and the lowest in the lower esophageal sphincter region. Duration of contraction is very short, less than a second. Propagation velocity of the pen staltic wave is fast. Although the llama esophagus is long, it takes only a few seconds for the peristaltic wave to travel from the proximal to the distal end of the esophagus. However, propagation velocity of a solid bolus is slower than that of the liquid bolus. Secondary peristalsis, a local peristalsis not initiated by swallowing, occurrs more frequently in the horizontal thoracic portion than the vertical cervical portion of the esophagus. The function of secondary peristalsis is to keep the esophagus clean. Without gravity and because of the close contact with Cl, failure of esophageal peristalsis may result in food accumulation in the thoracic portion of the esophagus. Length of the cervical portion is almost twice that of the thoracic portion of the esophagus in keeping with long neck of the llama. Mucous secreting submucosal 110 This study provides extensive normal data on the llama esophagus to serve as a baseline for further study of esophageal pathology in the llama. Findings from this study also suggest that megaesophagus in llamas can occur due to denervating disease. The exact nature of which is still unknown. ill BIBLIOGRAPHY Anderson NV, Vestweber JG. Hiatal hernia and segmental megaesophagus in a cow. J Am Vet Med Assoc 1984;184:193-195. Andrew BL. The nervous control of cervical esophagus of the rat. J Physiol 1956; 134:729-740. Asaad K, Rahman SA, Nawar NNY et al. Intrinsic innervation of the oesophagus in dogs with special reference to the presence of muscle spindles. Acta Anat 1983;1 15:91-96. Ask P, Tibbling L. Effect of time interval between swallows on esophageal peristalsis. Am J Physiol 1980;238:G485-G490. Bargai U, Nanthan AT, Pearl S. Acquired megaesophagus in a heifer. Vet Radiol 1991;32:259-260. Barone FC, Lombarcli DM, Ormsbee III HS. Effects of hindbrain stimulation on the lower esophageal sphincter pressure in the cat. Am J Physiol 1984;247:G70-G78. Berezin I, Daniel EE, Huizinga JD. Ultrastructure of interstitial cells of Cajal in the canine distal esophagus. Can J Physiol Pharmacol 1994;72:1049-1059. Berger SL, Palmer RH, Hodges CC, et al. Neurologic manifestations of trypanosomiasis in a dog. J Am Vet Med Assoc 1991;198:132-134. Bowman KF, Vaughan JT, Quick CB, et al. Megaesophagus in a colt. J Am Vet Med Assoc 1978;172:334-337. Boudrieu RJ, Rogers WA. Megaesophagus in the dog: A review of 50 cases. J Am Vet Med Assoc 1985;21:33-40. Brooke MH, Kaiser KK. Muscle fiber type: how many and what kind? Arch Neurol 1970;23:369-379. Brown FC, Gideon RM, Voelker FA, et al. Muscle function and structure of the esophagus of the baboon (Papio anubis). Am J Vet Res 1978;39:1209-1211. Burgess JN, Schlegel JF, Ellis FR. Effect of denervation on feline esophageal function and morphology. J Surg Res 1972;12:24. 112 Butt TD, Macdonald DG, Crawford WH. Persistent right aortic arch in a mature llama. VetRec 2001;148:118-119. Can DH, Scott PC, Titchen DA. Manometric and electromyographic observations of the oesophagus of sheep in eructation, regurgitation and swallowing. Quarterly Journal of Experimental Physiology 1983 ;68 :661-674. Castell JA, Castell DO. Stationary esophageal manometry. In: Scarpignato C, Galmiche JP,eds. Functional investigation in esophageal disease 1994: 109-129. Castell JA, Dalton CB, Castell DO. Pharyngeal and upper esophageal sphincter manometry in humans. Am J Physiol 1990;258:G173-G178. Castell JA, Gideon RM. Esophageal manometry. In Castell DO, Richter JE, eds. The Esophagus, 3d ed. Philadelphia: Lippincott Williams & Wilkins, 1999;101117. Christensen J, Rick GA, Robison BA, et al. Arrangement of the myentenc plexus throughout the gastrointestinal tract of the opossum. Gastroenterol 1983 ;85 :890899. Christensen J, Robison BA. Anatomy of the myenteric plexus of the opossum esophagus. Gastroenterol 1982; 83:1033-1042. Clark ES, Morris DD, Whitlock RH. Esophageal manometry in horses, cows, and sheep during deglutition. Am J Vet Res 1987;48:547-551. Clifford DH, Gyorkey F. Myenteric ganglial cells in dogs with and without achalasia of the esophagus. J Am Vet Med Assoc 1967;150;205-21 1. Cummings IF, DeLahunta A, Mitchell WJ. Ganglioradiculitis in the dog. Acta Neuropathol 1983;60:29-39. Darke PGG, Roberts TA, Smart JL, et al. Suspected botulism in foxhounds. Vet Rec 1976;99:98-100. Dellmann HD. Digestive system. In: Veterinary Histology. Philadelphia: Lea and Febiger, 1971;153-154. Dellmann DT, Brown EM. Digestive system. In: Textbook of Veterinary Histology. Philadelphia: Lea and Febiger, 1976;225-226. 113 DeNardi FG, Riddell RH. The normal esophagus. Am J Surg Pathol 1991;15:296309. Diamant NE. Neuromuscular mechanisms of primary peristalsis. Am J Med 1997; 103 :40s-43s. Dodds WJ et al. A comparison between primary esophageal peristalsis following wet and dry swallows. J AppI Physiol 1973;35:851-857. Dooley CP, Di Lorezo C, Valenzuela JE. Esophageal function in humans. Effect of bolus consistency and temperature. Dig Dis Sci 1990;35: 167-172. Duncan ID, Griffiths JR. Canine giant axonal neuropathy; some aspects of its clinical, pathological and comparative features. J Small Anim Pract 1981;22:491501. Faussone-Pellegrini MS, Cortesini C. Ultrastructure of striated muscle fibers in the middle third of the human esophagus. Histol Histopathol 1986;1: 119-128. Fawcett DW, Raviola E. The esophagus and stomach. In: Fawcett, ed. A Textbook of Histology, l2t ed. New York: Chapma & hall, 1994;593-616. Fowler ME. Digestive system. In: Medicine and Surgery of South American Camelids: Llama, Alpaca, Vicuna, Guanaco. 2x1 ed. Iowa: Iowa State University Press, 1998;305-359. Fryscak T, Zenker W, Kantner D. Afferent and efferent innervation of the rat esophagus: a tracing study with horseradish peroxidase and nuclear yellow. Anat Embryol 1984;170:63-70. Gaynor AR, Shofer FS, Washabau RJ. Risk factors for acquired megaesophagus in dogs. J Am Vet Med Assoc 1997;21 1:1406-1412. Getty R. Digestive system of the sheep. In: Sisson and Grossman's The Anatomy of the Domestic Animals, 5th ed. Philadelphia: WB Saunders Co, 1975;477-484. Goetsch E. The structure of the mammalian esophagus. Am J Anat 1910;10:1-40. Goyal RK, Sivarao DV. Functional anatomy and physiology of swallowing and esophageal motility. In: Castell DO, Richter JE, eds. The Esophagus, 3'' ed. Philadelphia: Lippincott Williams & Wilkins, 1999;1-32. 115 Jacobowitz D, Nemir P. The autonomic innervation of the esophagus of the dog. J Thorac and Cardiovasc Surg 1969 ;5 8:678-684. Jamdar MN, Ema AN. The submucosal glands and the orientation of the musculature in the oesophagus of the camel. J Anat 1982;135:165-171. Janssens J, Valembois P, Vantrappen G, et al. Is the primary peristaltic contraction of the canine esophagus bolus-dependent? Gastroenterology 1973;65:750-756. Johnston BT Collins JSA, McFarland RJ, et al. A comparison of esophageal motility in response to bread swallows and water swallows. Am J gastroenterol 1993;88:351-355. Kahrilas PJ. Functional anatomy and physiology of the esophagus. In: Castell DO, Richter JE, eds. The Esophagus 2' ed. Philadelphia: Lippincott-Reven, 1995;1-28. Kahrilas PJ, Clouse RE, Hogan WJ. American Gastroenterological Association technical review on the clinical use of esophageal manometry. Gastroenterology 1994;107:1865-1884. Kahrilas PJ, Dent J, Dodds WJ et al. A method for continuous monitoring of upper esophageal sphincter pressure. Dig Dis Sci 1987;32:121-128. Kaye MD, Wexler RM. Alteration of esophageal peristalsis by body position. Dig Dis Sci 198 1;26:897-901. Keren S, Argaman Golan M. Solid swallowing versus water swallowing: manometric study of dysphagia. Dig Dis Sci 1992;37:603-608. Komegay JN, Gorgacz EJ, Dawe DL, et al. Polymyositis in dogs. J Am Vet Med Assoc 1980;176:431-438. Krum SR. Cardinet GH, Anderson BC, et al. Polymyositis and polyarthritis associated with systemic lupus erythematosis in a dog. J Am Vet Med Assoc 1977; 170:61-64. Kuramoto H, Kato Y, Sakamoto H, et al. Galanin-containing nerve terminals that are involved in a dual innervation of the striated muscles of the rat esophagus. Brain Res 1996;734:186-192. Lawn AM. The localization, by means of electrical stimulation, of the origin and path in the medulla oblongata of the motor nerve fibers of the rabbit esophagus. J Physiol 1964;174:232-244. 116 Li Q, Castell JA, Caste!! DO. Manometric determination of esophageal length. Am J Gastroenterol 1994;89:722-725. Long JD, Orlando RC. Esophageal submucosal glands: structure and function. Am J Gastroenterol 1999;94:2818-2824. Maddison JE, Allan GS. Megaesophagus attributable to lead toxicosis in a cat. J Am Vet Med Assoc 1990;197:1357-1358. Marsden PD, Hagstrom JWC. Experimental Trypanosoma cruzi infection in beagle puppies. Trans R Soc Trop Med Hyg 1968;62:816-824. Mascarello F, Rowlerson A, Scapolo PA. The fibre type composition of the striated muscle of the esophagus in ruminants and carnivores. Histochemistry 1984;80: 277-288. Mason KV. A case of myasthenia gravis in a cat. J Small Anim Pract 1976;17:467472. McGavin MD, Valentine BA. Muscle. In: McGavin MD, Canton W, Zachary, JF, eds. Thomson's Special Veterinary Pathology. St. Louis: Mosby mc, 2000;461498. Meyer GW, Austin RM, Brady CE, et al. Muscle anatomy of the human esophagus. J Clin Gastroenterol 1986;8:131-134. Miller LM, Lennon VA, Lambert EH, et al. Congenital myasthenia gravis in 13 smooth Fox Terriers. J Am Vet Med Assoc 1983;182:694-702. Morikawa S, Komuro T. Distribution of myentenic NO neurons along the guineapig esophagus. J Auton Nerv Syst 1998;74:91-99. Monikawa S, Komuro T. Ultrastructure of intramural ganglia in the striated muscle portions of the guinea pig esophagus. J Anat 1999;195: 111-120. Murray MJ. The esophagus. In: Reed SM, Bayly WM, eds. Equine Internal Medicine. Philadelphia: WB Saunders Co, 1998;608. Murray MJ, Ball MM, Parker GA. Megaesophagus and aspiration pneumonia secondary to gastric ulceration in a foal. J Am Vet Med Assoc 1988;192:381-383. 117 Neuhuber WL, Eichhorn U, Won J. Enteric co-innervation of striated muscle fibers in the esophagus: just a "hangover"? Anat Rec 2001;262(1):41-46. O'Brien GM. Histochemistry of the muscularis extema of the oesophagus in rabbit. Proc Austr Physiol Pharm Soc 1982;13:23P. Orlando RC. Pathophysiology of gastroesophageal reflux disease. In Cstell DO, Richter JE, eds. The esophagus. Philadelphia: Lippincott Willams &Wilkins, 1999;409-419. Parish SM, Middleton JR, Baldwin TJ. Clinical megaesophagus in a goat with thymoma. VetRec 1996;139:94. Patapoutian A, Wold BJ, Wagner RA. Evidence for developmentally programmed transdifferentiation in mouse esophageal muscle. Science 1995;270: 1818-1820. Paterson WG, Rattan 5, Goyal RK. Esophageal response to transient and sustained esophageal distention. Am J Physiol 1988;255:G587. Pflugfelder CM, Cardinet GH, Lutz H, et al. Acquired canine myasthenia gravis: immunocytochemical localization of immunes complexes at neuromuscular junctions. Muscle Nerve 1981; 4: 289-295. Randelia HP, Lalitha VS. Megaesophagus in ICRC mice. Lab Anim 1988;22:23-26. Richter JE, Wu WC, Johns DN et al. Esophageal manometry in 95 healthy adult volunteers. Dig Dis Sci 1987;32:583-592. Roman C, Gonella J. Extrinsic control of digestive tract motility. In Johnson LR, ed, Physiology of the gastrointestinal tract 92nd ed). New York: Raven Press, 1987:507. Ross CE, Rebhun WC. Megaesophagus in a cow. J Am Vet Med Assoc 1986;1 88:623-624. Samarasinghe DD. Some observations on the innervation of the striated muscle in the mouse oesophagus - an electron microscope study. J Anat 1972;112:173-184. Sang Q, Young HM. Development of nicotinic receptor clusters and innervation accompanying the change in muscle phenotype in the mouse esophagus. J Comp Neurol 1997; 386:119-136. 118 Satchel! PM. The neuropathic oesophagus. A radiographic and manometric study on the evolution of megaesophagus in dogs with developing axona! neurophathy. Res Vet Sci 1990;48:249-255. Schummer A, Nickel R, Sack WO. The alimentary canal. In: The Viscera of Domestic Mammals, New York: Springer-Verlag, 1979;99-202. Sears VW Jr, Castell JA, Castell DO. Comparison of effects of upright versus supine body position and !iquid versus solid bolus on esophageal pressures in normal humans. Dig Dis Sci 1990;35:857. Shedlofsky-Deschamps G, Krause WJ, Cutts JH et al. Histochemistry of the striated musculature in the opossum and human oesophagus. J Anat 1982;134:407414. Shell LG, Jortner BS, Leib MS. Spinal muscular atrophy in two Rottweiler littermates. J Am Vet Med Assoc 1987;190:878-880. Shelton GD, Schule A, Kass PH. Risk factors for acquired myasthenia gravis in dogs: 1,154 cases (1991-1995). JAm Vet Med Assoc 1997;211:1428-1431. Shelton GD, Willard MID, Cardinet GH, et al. Acquired myasthenia gravis: selective involvement of esophageal, pharyngeal, and facial muscle. J Vet Intern Med 1990; 4: 28 1-284. Sifrim D, Janssens J, Vantrappen G. A wave of inhibition precedes primary peristaltic contractions in the human esophagus. Gastroenterology 1992; 103:876882. Slocombe RF, Todhunter RJ, Stick JA. Quantitative ultrastructural anatomy of esophagus in different regions in the horse; effects of alternate methods of tissue processing. Am J Vet Res 1982;43: 1137-1142. Smith BB. Natural history and industry overview. Camelid medicine and surgery. Revision 3.71. 1998: 1.1-1.9. Stef JJ, Dodds WJ, Hogan, et al. Intraluminal esophageal manometry: an analysis of variables affecting recording fidelity of peristaltic pressures. Gastroenteology 1974;67: 221-230. Stevens CE, Sellers AF. Pressure events in the bovine esophagus and reticulorumen associated with eructation. Am J Physiol 1960;199:598-602. 121 Zook BC, Gilmore CE. Thallium poisoning in dogs. J Am Vet Med Assoc 1967;151:206-217.