Hemangioblastoma Neoplasm of FLAIR MRI Confront in Vigorous Dissection B.Rajesh Kumar
advertisement
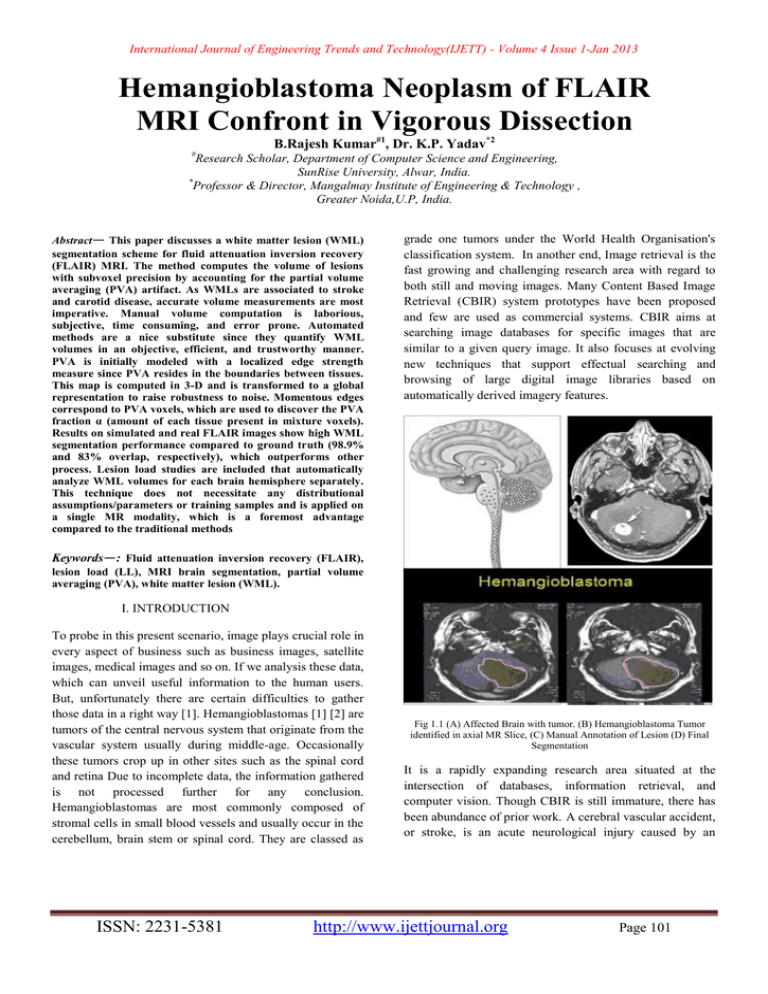
International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 Hemangioblastoma Neoplasm of FLAIR MRI Confront in Vigorous Dissection B.Rajesh Kumar#1, Dr. K.P. Yadav*2 # Research Scholar, Department of Computer Science and Engineering, SunRise University, Alwar, India. * Professor & Director, Mangalmay Institute of Engineering & Technology , Greater Noida,U.P, India. Abstract— This paper discusses a white matter lesion (WML) segmentation scheme for fluid attenuation inversion recovery (FLAIR) MRI. The method computes the volume of lesions with subvoxel precision by accounting for the partial volume averaging (PVA) artifact. As WMLs are associated to stroke and carotid disease, accurate volume measurements are most imperative. Manual volume computation is laborious, subjective, time consuming, and error prone. Automated methods are a nice substitute since they quantify WML volumes in an objective, efficient, and trustworthy manner. PVA is initially modeled with a localized edge strength measure since PVA resides in the boundaries between tissues. This map is computed in 3-D and is transformed to a global representation to raise robustness to noise. Momentous edges correspond to PVA voxels, which are used to discover the PVA fraction α (amount of each tissue present in mixture voxels). Results on simulated and real FLAIR images show high WML segmentation performance compared to ground truth (98.9% and 83% overlap, respectively), which outperforms other process. Lesion load studies are included that automatically analyze WML volumes for each brain hemisphere separately. This technique does not necessitate any distributional assumptions/parameters or training samples and is applied on a single MR modality, which is a foremost advantage compared to the traditional methods grade one tumors under the World Health Organisation's classification system. In another end, Image retrieval is the fast growing and challenging research area with regard to both still and moving images. Many Content Based Image Retrieval (CBIR) system prototypes have been proposed and few are used as commercial systems. CBIR aims at searching image databases for specific images that are similar to a given query image. It also focuses at evolving new techniques that support effectual searching and browsing of large digital image libraries based on automatically derived imagery features. Keywords—: Fluid attenuation inversion recovery (FLAIR), lesion load (LL), MRI brain segmentation, partial volume averaging (PVA), white matter lesion (WML). I. INTRODUCTION To probe in this present scenario, image plays crucial role in every aspect of business such as business images, satellite images, medical images and so on. If we analysis these data, which can unveil useful information to the human users. But, unfortunately there are certain difficulties to gather those data in a right way [1]. Hemangioblastomas [1] [2] are tumors of the central nervous system that originate from the vascular system usually during middle-age. Occasionally these tumors crop up in other sites such as the spinal cord and retina Due to incomplete data, the information gathered is not processed further for any conclusion. Hemangioblastomas are most commonly composed of stromal cells in small blood vessels and usually occur in the cerebellum, brain stem or spinal cord. They are classed as ISSN: 2231-5381 Fig 1.1 (A) Affected Brain with tumor. (B) Hemangioblastoma Tumor identified in axial MR Slice, (C) Manual Annotation of Lesion (D) Final Segmentation It is a rapidly expanding research area situated at the intersection of databases, information retrieval, and computer vision. Though CBIR is still immature, there has been abundance of prior work. A cerebral vascular accident, or stroke, is an acute neurological injury caused by an http://www.ijettjournal.org Page 101 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 interruption of the vascular supply of blood to the brain. Since blood is a carrier of nutrients and oxygen, the pretentious neurons begin to die within minutes due to oxygen and/or nutrient starvation (ischemic stroke) [1]. Stroke can result in significant neurological deficits, leading to various physical impairments such as sensory motor paralysis, loss of sensation and motor control, as well as complexity in interpreting spatial relationships [1]; stroke can also be fatal. According to the Canadian Heart and Stroke Society, about 50 000 Canadians endure new or recurrent strokes each year, which on average means a stroke occurs every 10 min. It is the third cause of death behind heart disease and cancer and costs the Canadian economy roughly $3.6 billion a year in physician services, hospital costs, lost wages, and decreased productivity [2]. MRI is superior to nonenhanced CT in the detection of vascular components of the tumor. [8, 10] Contrastenhanced CT has the same sensitivity as nonenhanced MRI; though, it is inferior to contrast-enhanced MRI. [8] Contrast-enhanced MRI permits the identification of small tumor nodules. In addition, MRI is helpful in unraveling cystic and solid components of the tumor from edema. Patients with VHL should be screened, and follow-up studies should be carried out at 6 months. The sensitivity of MRI increases with the use of gadolinium-based contrast material. Angiography is better in the detection of small (< 1 cm) vascular tumor components, and it is enhanced for showing the vascular nature, supply, and drainage of tumors, compared with CT.[11] Though, CT and MRI depict tumor cysts better.[12]. Use of a gadolinium-based contrast agent is mandatory in the evaluation of hemangioblastomas because it increases the sensitivity for small, solid lesions. [11, 13] When the diagnosis of hemangioblastoma is established, a cautious evaluation for other petite enhancing lesions should be performed because of the multiple lesions seen in some patients. The presence of multiple lesions has an important impact on the prognosis. [6]. In hemangioblastomas located near the pia, differentiation from meningiomas can be difficult in certain patients. It is always imperative to look for characteristics that can aid in diagnosing a vascular tumor, which is particularly important before surgery. In some patients, complete removal of the mural nodule is enough; though, in patients in whom the transition from solid tumor to cystic tumor with a mural nodule has been observed, the radiologist should perform careful follow-up imaging to assess the behavior of the lesions. To reduce the mortality rates and long-term disabilities associated with stroke, physicians are investigating magnetic resonance images (MRI) of the brain to determine if precursors exist. Identifying early on stages of the disease can lead to new intervention protocols and therapeutic strategies which ISSN: 2231-5381 ultimately may reduce the incidence of stroke. MRI are fast becoming the de facto standard for brain analysis since MR scanners generate high-resolution images with nonionizing radiation and they offer a noninvasive view of the softtissue structures of the brain. Through the analysis of a specific type of MRI, known as fluid attenuation inversion recovery (FLAIR), researchers have found that abnormal changes in the white matter, known as white matter lesions (WML), are a surrogate for future stroke [3]. FLAIR images have similar tissue contrasts as T2-weighted MRI (suppressed fat signal, hyper intense in water-based tissues), except that the cerebrospinal fluid (CSF) signal is nulled for enhanced discrimination of ischemic pathology [4]. Resultantly, WML appear as hyper intense objects scattered throughout the white matter in FLAIR MRI. Using the FLAIR images, lesions were manually outlined by a specialist and the volumes were found based on the number of pixels in the region. The volumes of these lesions [lesion load (LL)] were important markers in determining their relationship to stroke. Since there is evidence that relates WML to ischemic stroke, there is growing interest and research efforts dedicated to understanding the phenomena of this WML generation. Discovery of non-genetic and nonage-dependant factors of WML could aid in the development of new intervention protocols or therapies (for the mechanism which causes WML), ultimately reducing a patient’s risk of stroke. II. LITERATURE SURVEY Segmentation Image retrieval is the basic requirement task in the present scenario. Content Based Image Retrieval is the popular image retrieval system by which the target image to be retrieved based on the useful features of the given image. In other hand, image mining is the arising concept which can be used to extract potential information from the general collection of images. Target or close Images can be retrieved in a little fast if it is clustered in a right manner. In this paper, the concepts of CBIR and Image mining have been combined and a new clustering technique has been introduced in order to increase the speed of the image retrieval system. Classification followed by mathematical morphology. Level set evolution with constant propagation needs to be initialized either completely inside or outside the tumor and can leak through weak or missing boundary parts. Replacing the constant propagation term by a statistical force overcomes these limitations and results in a convergence to a stable solutionIn fuzzy classifier systems the classification is obtained by a number of fuzzy If} Then rules including linguistic terms such as Low and High that fuzzy each feature. This paper presents a scheme by which a reduced linguistic (fuzzy) set of a labelled multi-dimensional data http://www.ijettjournal.org Page 102 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 set can be identified automatically. After the projection of the original data set onto a fuzzy space, the optimal subset of fuzzy features is determined using conventional search techniques. The applicability of this method has been demonstrated by reducing the number of features used for the classification of four real-world data sets. This scheme can also be used to generate an initial rule set for a fuzzy neural network III. METHODOLOGY 3.1 Existing Method Through the analysis of a specific type of MRI, known as fluid attenuation inversion recovery (FLAIR), researchers have found that abnormal changes in the white matter, known as white matter lesions (WML), are a surrogate for future stroke [3]. FLAIR images have similar tissue contrasts as T2-weighted MRI (suppressed fat signal, hyper intense in water-based tissues), except that the cerebrospinal fluid (CSF) signal is nulled for enhanced discrimination of ischemic pathology [4]. Resultantly, WML appear as hyper intense objects scattered throughout the white matter in FLAIR MRI. Using the FLAIR images, lesions were manually outlined by a specialist and the volumes were found based on the number of pixels in the region. The volumes of these lesions [lesion load (LL)] were important markers in determining their relationship to stroke. Since there is evidence that relates WML to ischemic stroke, there is growing interest and research efforts dedicated to understanding the phenomena of this WML generation. Discovery of non-genetic and nonage-dependant factors of WML could aid in the development of new intervention protocols or therapies (for the mechanism which causes WML), ultimately reducing a patient’s risk of stroke. These medical studies that examine the relationship between WML and stroke or carotid disease are completed by humans. Therefore, the number of patients that can be included in the study is limited (specialists must outline lesions from several slices per patient, which is a daunting task). With small sample sizes, it is difficult to study longterm effects or the “true” statistical nature for large patient cohorts. Furthermore, as with any human-based analysis, manually acquiring the volumes of the lesions is subjective (observer dependant), time consuming, laborious, and error prone. Automated techniques are a great alternative since they can automatically segment the WML and compute their volume in a quantitative, efficient, reproducible, and trustworthy manner. Any numeral of images can be included in the study and LLs can be obtained within minutes. large vessel atherosclerosis and WML [5]. In [6], Moody et al. examine the relationship between WML and carotid plaque morphology as an indirect measure of micro embolic activity. The hypothesize that if micro embolisms from complicated plaques are a contributing factor to WML, then these lesions should be more frequent and evident in the ipsilateral cerebral hemisphere (same side of the body as the diseased carotid). To test this hypothesis, WML were manually segmented from FLAIR images of the brain and the volumes of the lesions from each of the brain’s hemispheres were computed. Statistical tests found twice as many WML (higher LL) in the brain hemisphere that is on the same side of the body (ipsilateral) to complicated carotid plaques. Similar studies have shown the same results [5]. Most works for WML segmentation focus on intensity clustering of multispectral datasets, (T1, T2, PD, FLAIR) [7]–[11]. In [7] and [8], the authors examine the clusterability of image classes for intensity-based feature vectors defined from the coregistered dataset. Although promising, the need for protracted multiparametric scanning reduces the appeal of such approaches. Acquisition of several volumes per patient is expensive, dependent on the performance of a registration algorithm (which can introduce errors for over- and under-fitting) and results in images that are prone to motion artifacts (patients spend more time in the MR scanner). Moreover, the use of many images increases memory requirements and the computational complexity of the algorithm. 3.3 PROPOSED METHOD There are many works that deal with segmentation of multiple sclerosis (MS) plaques or lesions in FLAIR MRI. For example, the works in [12] and [13] use a Bayesian classifier with the adaptive mixtures method and Markov random field classifier to automatically estimate and update the class conditional probability density function and the a priori probability of each tissue class. MS plaque segmentations were validated extensively with manual segmentations and the results are very promising. Although MS lesions have similar appearance to WML in FLAIR MRI in many instances, MS lesions can have unique characteristics that set them apart from WML. Not only the origin of these diseases is different (MS is an inflammatory demyelinating disease [14], whereas WML are selective incomplete white matter infarction [6]), the location of the lesions can also differ substantially. For example, juxtacortical lesions (inside the cortex) are specific to MS and not WML [14]. Consequently, not all MS segmentation schemes may be applicable for WML detection and quantification. 3.2 Disadvantages Obtaining memberships partitioned The etiology or pathogenesis of WML is still largely debated. For some time, the predominant notion was that WML is strongly associated with small vessel disease. However, as of more recently, there has been an accumulation of evidence that supports the link between As will be shown, the parameter α dictates how much of each tissue is present in a voxel. Consequently, PVA quantification is usually posed as an estimation problem, where this variable α is sought. Traditional techniques for normal T1 or T2 MRI search for a global estimate of this ISSN: 2231-5381 http://www.ijettjournal.org Page 103 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 parameter, i.e., α(y), which quantifies the proportion of one tissue present in a voxel of intensity y [16], [17]. Unfortunately, previous model-based methods are not directly applicable since pathology modifies the intensity distribution in a manner that is difficult to model. Moreover, neurological MRI often has non-Gaussian or unknown noise properties, causing techniques that rely on normality to be inaccurate [18]. To combat these downfalls, this study focuses on a nonstatistical, image-based PVA modeling approach for robust segmentation of WML in FLAIR. The following section details the methods used. 3.4 Algorithm To prove the efficacy of the proposed method, both simulated and real images with ground truths are used. To objectively validate the performance of the proposed method on these databases, the amount of intersection between a segmented object and the gold standard is measured by the dice similarity coefficient (DSC): ( | ( ) ⋂ ( )| | ( )| | ( )| ) ( )) 3.5 Flair On Implementation 1) Simulated Data: A series of FLAIR images with WML are simulated based on McGill’s Brainweb database. Brainweb contains a series of T1/T2/PD images (normal and MS lesions) with ground truth masks for GM, WM, CSF, and MS lesion classes. Several Brainweb slices with MS lesions that have similar appearance and spatial location as WML are used for simulation purposes. To generate FLAIR images, the WM and GM classes are joined, resulting in three pure tissue classes: CSF, brain (GM and WM), and WML. This mask is interpolated by two to double the size of the mask. 2) Real Data: To validate the algorithm on real data, a total of 25 images were selected by randomization from the database. The images were chosen according to uniform sampling without replacement. The validation database was given to an expert radiologist (AM) for the manual WML segmentations (ground truth). AM was blinded to all patient ISSN: 2231-5381 Advantages As WML have been shown to be related to carotid disease ipsilaterally, large differences in the hemispheric LLs could be an indication of advanced carotid disease. Preliminary results presented here on the patient data demonstrate how the proposed works can be used to gather statistics regarding WML automatically, efficiently, and reliably. The end goal of these methods is WML segmentation in large studies to further examine the relationship between WML, stroke, and carotid disease. Therefore, we are currently recruiting more patients and preparing to apply this research on a large patient cohort. However, the methods presented here give a clear sense of direction and show the potential of automated analysis for WML segmentation. IV.WHITE MATTER NEOPLASM DISSECTION Where A(x) and B(x) are binary masks for the segmentation and ground truth. Since the DSC only highlights the amount of overlap between two sets, to further quantify the efficiencies and deficiencies of the proposed method, specificity and sensitivity are used. This line divides the ROC space, where points above this line represent good classification, whereas points below this line indicate poor performance. Finally, the volumes of the lesions are computed with (∑ information, as well as the segmentation results. The software used to draw the regions of interest is known as the Sedeen Viewer.5 It allows users to easily load images, draw contours for desired regions of interest, as well as manual editing of the drawn contours. As will be shown, the parameter α dictates how much of each tissue is present in a voxel. Consequently, PVA quantification is usually posed as an estimation problem, where this variable α is sought. Traditional techniques for normal T1 or T2 MRI search for a global estimate of this parameter, i.e., α(y), which quantifies the proportion of one tissue present in a voxel of intensity y [16], [17]. Unfortunately, previous model-based methods are not directly applicable since pathology modifies the intensity distribution in a manner that is difficult to model. Moreover, neurological MRI often has non-Gaussian or unknown noise properties, causing techniques that rely on normality to be inaccurate [18]. To combat these downfalls, this study focuses on a nonstatistical, image-based PVA modelling approach for robust segmentation of WML in FLAIR. The following section details the methods used List of Modules 1. PVA Model 2. Edge-Based PVA Modeling 3. Fuzzy Edge Model 4. Global Edge Description 5. Estimating α http://www.ijettjournal.org Page 104 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 PVA Model compute this metric, the traditional magnitude of the gradient, i.e., g, is first estimated by PVA generates an image intensity which is linearly dependant on the proportion of each tissue in the voxel. In neuro MRI, where two tissue types mix per PVA voxel [17], the intensities of these mixels,1 Yjk (x), are determined by the proportion of the first tissue j, in comparison to that of the second tissue k, as in ( ) ( ) ( ) ( ( )) ( ) where Yj (x) is the intensity value drawn from first tissue’s intensity distribution pj (y) at spatial location x = (x1, x2 ) ∈ Z2 , Yk (x) is the intensity of the second tissue ∼pk (y), andα ∈ [0, 1] is the proportion of tissue j present at x (the remainder of the voxel is a fraction of tissue k, i.e., 1 − α). Using this mathematical relationship that describes PVA in terms of the intensities of mixtures voxels, we will quantify PVA in a new way based on the edge content of the image. Edge-Based PVA Modeling To examine the edge content in the PVA regions, consider the ideal signal model. Since edge content is described by the gradient, the gradient of these equations are taken resulting in ( ) ( ) Where α is the change in the proportion of tissues parameter (dictates how the proportion of one tissue changes as a function of space). Solving for the change in the proportion of tissues that result in two PVA quantifiers. Each PVA measure α jk is a normalized, class-specific representation of edge information in PVA regions. It is a normalized representation because the largest possible value of Y jk is Ij − Ik (maximum intensity change in one pixel step) and the minimum is 0 in a constant region, resulting in 0 ≤ αjk ≤ 1. Since these class-specific variables describe PVA in terms of the gradient, this study focuses on an edgebased estimate for αand uses it to decode the proportion of tissues parameter α. Fuzzy Edge Model To estimate α(x), a fuzzy technique based on the cumulative distribution function (CDF) of the gradient is employed. To ISSN: 2231-5381 ‖ √| ‖ | | | Where the Sobel operator is used. The probability distribution function (PDF) of the gradient pG (g) is computed next, and based on this PDF, the CDF of the gradient magnitude is found and used as an estimate for the edge information in the image. ( ) ( ) ∑ () Where α(g) ∈ [0, 1]. This nonlinear fuzzification of the edge information quantifies the “certainty of edge presence.” Note that this parameter is expressed as a function of the gradient and to be used to approximate α(x), α(g) is mapped back to the spatial domain: α(g) → α(x). This fuzzy edge measure assigns large and similar values to significant edges, despite them occurring over a wide range of g and with few occurrences. It groups significant edges, while suppressing the irrelevant ones. As this edge measure is normalized, i.e., 0 ≤ α(x) ≤ 1, and representative of the edge information in the image, it is used to represent PVA in the image. Global Edge Description PVA occurs over specific intensity ranges, and moreover, with high edge values. These two features, intensity and edge strength, are coupled together in the following section to arrive at a new and denoized version of the fuzzy edge metric. Initially, edge and intensity information is coupled through the conditional PDF of α(x), for a particular intensity y, by ( )| ( ( ) | ) ( ) | Where 0 ≤ a ≤ 1, 0 ≤ y ≤ ymax, a is the realization of α(x), and y max is the maximum gray level in the image. This PDF quantifies the distribution of the edge information α(x) for a specific gray level y. Because it was computed on the entire image (or volume for 3-D approaches), it describes the global clustering trend of edge information as a function of intensity. Generally, in flat regions (pure tissues) there is clustering in the PDF for low edge values at corresponding intensities. Across anatomical boundaries (PVA), high edge. http://www.ijettjournal.org Page 105 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 Estimation of the PDF this way automatically classifies the voxel y as belonging to either the pure tissue or PVA class. Pure regions correspond to maxima in p (a = 0|y) and minima in p (a = 1|y), whereas minima in p (a = 0|y) and maxima in p (a = 1|y) indicate with high likelihood that these voxels belong to a PVA region. To determine the global estimate of α(y), the conditional expectation operator is used. It offers the best prediction of α given that the intensity is y in the mean squared error sense. The result is an enhanced edge map α(y), ( ) ( )| ∑ ( )| ( )| ( ( | ) ( | ) ( )| ( | ) | ) Which provides a global representation of the edge information in the image indicating that the quantification of PVA content is directly proportional to the probability that a voxel is located on an edge. Estimating α To decode α(y), regions of α(y) are retained, while others discarded. Recall that the maxima of p (a = 1|y) dictate which voxels y are most likely PVA (maximally edgy), while the minima are correlated with voxels y from pure tissue classes (minimally edgy or flat). Ideally, in flat regions (pure tissues), there should be no edge information, but noise generates “artificial” edginess, causing the minima of α(y) to be nonzero in these regions. To account for the relative nature of α(y), an adaptive threshold is applied. Where tL and tR are the left and right thresholds, respectively, and mink is the minimum of α(y) corresponding to tissue k, minj is the minimum of α(y) corresponding to pure tissue j, and maxjk is the maximum of the PVA pulse that describes the mixture of tissues j and k. The minima and maxima values are easily found with a peak-finding algorithm, which uses derivative information to find optima. V. EXPERIMENT RESULT The programming language used with MATLAB is usually referred to as MATLAB script or M-script. After becoming familiar with the basic syntax of the M-script, a number of useful utilities are available to you that allow you to make extended uses of MATLAB. You can, for example, write programs that involve simulation. You can also create graphics, web pages, and GUI applications. When you develop programs using MATLAB, you can output the results to a number of media, including graphics files, HTML pages, PDF files, and Word documents. You can also connect up MATLAB with other applications, such as Excel or Lab View to make extended uses of it. Since it is programmed in part using Java, you can modify it in the background using Java. The feasibility of the project is analyzed in this phase and business proposal is put forth with a very general plan for the project and some cost estimates. During system analysis the feasibility study of the proposed system is to be carried out. This is to make sure that the proposed system is not a burden to the company. For feasibility analysis, some understanding of the major requirements for the system is essential. SCREENSHOTS INPUT IMAGE An adaptive threshold that retains voxels most likely (in a probabilistic sense) to contain mixture components are computed for the left and right side of each PVA pulse ISSN: 2231-5381 http://www.ijettjournal.org Page 106 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 Fig: 5.3 Manual Annotations INITIAL ITERATION Fig: 5.1 Input MR Image 7.2 SEGMENTED IMAGE BASED ON ALGORITHM PROCESSING IMAGE FIGURE 1 Fig: 5.4 Computing the points FINAL ITERATION Fig: 5.2 Point Analysis PROCESSING STAGE Fig: 5.5 Segmenting the tumor region ISSN: 2231-5381 http://www.ijettjournal.org Page 107 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 TUMOR REGION EXTRACTION PROCESSING Fig: 5.6: Segmented tumor region Fig: 5.9 Point Analysis INITIAL ITERATION Fig: 5.7 Segmented tumors in surf analysis 7.3 SEGMENTED IMAGE BASED ON ALGORITHM PROCESSING IMAGE FIGURE 2 Fig: 5.10 Manual Annotation FINAL ITERATION Fig: 5.8 Initial Stage Of Another Image Fig: 5.11 Computing the points ISSN: 2231-5381 http://www.ijettjournal.org Page 108 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 CELL REGION EXTRACTION hemispheres of the brain separately, which has the potential to advance medical research on stroke and carotid disease. 6.2 FUTURE WORKS Preliminary results presented here on the patient data demonstrate how the proposed works can be used to gather statistics regarding WML automatically, efficiently, and reliably. The end goal of these methods is WML segmentation in large studies to further examine the relationship between WML, stroke, and carotid disease. It is most important that large databases are included to gain true knowledge of the underlying phenomena, and therefore, we are currently recruiting more patients and preparing to apply this research on a large patient cohort. Though, the methods presented here give a clear sense of direction and show the potential of automated analysis for WML segmentation. Fig: 5.12 Segmented cell region SURF ANALYSIS OF THE PROCESSED IMAGE REFERENCE [1] F. H. Martini, Fundamentals of Anatomy and Physiology, 5th ed. Fig: 5.13 Segmented cell region in surf VI. CONCLUSION 6.1 CONCLUSION This paper proposes a novel PVA quantification scheme that not only robustly segments WML in FLAIR MRI, but the other tissue classes as well. It focuses on a global edgebased approach since PVA voxels reside in boundaries between tissues. Results for simulated images show excellent results (WML, brain, and CSF are segmented with an accuracy of 98.5%, 99.9%, and 98.9%, respectively). Results on real images further show the utility of the work, as the WML segmentations show high correlation with the expert segmentations (average overlap of 83%). LL studies on a patient database show that the technique can be used to measure the volume of the WML in the left and right ISSN: 2231-5381 Englewood Cliffs, NJ: Prentice-Hall, 2001. [2] Canadian Hearth and Stroke Association. (2011). “Stroke statistics,” World Wide Web, [Online]. Available: http://www.heartandstroke.com [3] A. J. Fox, V. C. Hachinski, H. J. M. Barnett, J. Y. Streifler, M. Eliasziw, O. R. Benavente, and S. Alamowitch, “Prognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosis,” Stroke, vol. 33, pp. 1651–1655, 2002. [4] P. Malloy, S. Correia, G. Stebbins, and D. H. Laidlaw, “Neuroimaging of white matter in aging and dementia,” Clin. Neuropsych., vol. 21, pp. 73– 109, 2007. [5] R. E. Murphy, A. R. Moody, P. S. Morgan, and A. L. Martel, “Brain white matter hyperintensities are associated with carotid intraplaque hemorrhage,” Radiology, vol. 31, no. 1, pp. 202–209, 2007. [6] N. Altaf, L. Daniels, P. Morgan, J. Lowe, J. Gladman, S. MacSweeney, A. Moody, and D. Auer, “Cerebral white matter hyper intense lesions are associated with unstable carotid plaques,” Eur. J. Vasc. and Endovasc. Surg., vol. 31, pp. 8–13, 2006. [7] Z. Lao, D. Shen, A. Jawad, B. Karacali, D. Liu, E. Melhem, R. Bryan, and C. Davatzikos, “Automated segmentation of white matter lesions in 3D brain MRI, using multivariate pattern classification,” in Proc. IEEE Int. Symp. Biomed. Imaging (ISBI), 2006, pp. 307–310. [8] P. Anbeek, K. L. Vincken,M. J. van Osch, R. H. Bisschops, and J. van der Grond, “Automatic segmentation of different-sized white matter lesions by voxel probability estimation,” Med. Image Anal., vol. 8, no. 3, pp. 205– 215, 2004. [9] P. Anbeek, K. Vincken, M. van Osch, R. Bisschops, and J. van der Grond, “Probabilistic segmentation of white matter lesions in MR imaging,” NeuroImage, vol. 21, no. 3, pp. 1037–44, 2004. [10] R. de Boer, F. van der Lijn, H. A. Vrooman, M.W. Vernooij, M. A. Ikram, M. M. Breteler, and W. J. Niessen, “Automatic segmentation of brain tissue and white matter lesions in MRI,” in Proc. IEEE Int. Symp. Biomed. Imag., 2007, pp. 652–655. [11] R. de Boer, H. A. Vrooman, F. van der Lijn,M.W. Vernooij, M. A. Ikram, A. van der Lugt, M. M. Breteler, and W. J. Niessen, “White matter lesion extension to automatic brain tissue segmentation on MRI,” Neuroimage,vol. 45, no. 4, pp. 1151–1161, 2009. [12] R. Khayatia, M. Vafadusta, F. Towhidkhaha, and S.M. Nabavib, “Fully automatic segmentation of multiple sclerosis lesions in brain MR flair images using adaptive mixtures method and Markov random field model,” Comput. Biol. Med., vol. 38, no. 3, pp. 379– 390, 2008. [13] R. Khayatia, M. Vafadusta, F. Towhidkhaha, and S. M. Nabavib, “A novel method for automatic determination of different stages of multiple sclerosis lesions in brain MR flair images,” Comput. Med. Imag. Graph., vol. 32,no. 2, pp. 124–133, 2008. http://www.ijettjournal.org Page 109 International Journal of Engineering Trends and Technology(IJETT) - Volume 4 Issue 1-Jan 2013 [14] F. Barkhof and R. Smithuis. (2007). “Multiple sclerosis,” Radiology Assistant, [Online]. Available: http://www.radiologyassistant.nl/en/4556dea65db62 [15] C. R. Jack, P. C. O’Brien, D. W. Rettman, M. M. Shiung, Y. C. Xu, R. Muthupillai, A. Manduca, R.Avula, and B. J. Erickson, “Flair histogram segmentation for measurement of leukoaraiosis volume,” J. Mag. Res. Img., vol. 14, no. 6, pp. 668–676, 2001. [16] K. V. Leemput, F. Maes, D. Vandermeulen, and P. Suetens, “A unifying framework for partial volume segmentation of brain MR images,” IEEE Trans. Med. Imag., vol. 22, no. 1, pp. 105–119, Jan. 2003. [17] M. A. Gonz´alez Ballester, A. P. Zisserman, and M. Brady, “Estimation of the partial volume effect in MRI,” Med. Image Anal., vol. 6, no. 4, pp. 389–405, 2002. [18] A. Khademi, D. Hosseinzadeh, A. Venetsanopoulos, and A. R. Moody, “Nonparametric statistical tests for exploration of correlation and nonstationarity in images,” in Proc. Int. Conf. Digital Signal Process., 2009, pp. 1–6. [19] A. Khademi, A. Venetsanopoulos, and A. R. Moody, “Automatic contrast enhancement of white matter lesions in FLAIR MRI,” in Proc. IEEE Int. Symp. Biomed. Imag., 2009, pp. 1–4. [20] P. Meer and B. Georgescu, “Edge detection with embedded confidence,” IEEE Trans. Pattern Anal. Mach. Intell., vol. 23, no. 12, pp. 1351–1365, Dec. 2001. [21] A. Khademi, A. Venetsanopoulos, and A. Moody, “Edge-based PVA estimation in FLAIR MRI with WML,” in Proc. IEEE Eng. Med. Biol. Soc. Conf., 2010, pp. 6114–6117. [22] P. H. Kvam and B. Vidakovic, Nonparametric Statistics with Applications to Science and Engineering. New York: WileyInterscience, 2007. [23] A. Popovic, M. de la Fuente, M. Engelhardt, and K. Radermacher, “Statistical validation metric for accuracy assessment in medical image segmentation,” Int. J. Comput. Assist. Radiol. Surg., vol. 2, pp. 169–181, 2007. [24] C. Lisanti, C. Carlin, K. P. Banks, and D.Wang, “NormalMRI appearance and motion-related phenomena of CSF,” Amer. J. Roentgenol., vol. 188, no. 3, pp. 716–725, 2007. [25] Vijayakumar, B., and Ashish Chaturvedi. "Automatic Brain Tumors Segmentation of MR Images using Fluid Vector Flow and Support Vector Machine." Research Journal of Information Technology 4. [26] Vijayakumar, B., and Ashish Chaturvedi. "Tumor Cut-Segmentation and Classification of MR Images using Texture Features and Feed Forward Neural Networks." European Journal of Scientific Research 85.3 (2012): 363-372 [27] Vijayakumar, B., and Ashish Chaturvedi, “Idiosyncrasy Dissection and Assorting of Brain MR Images Instigating Kernel Corroborated Support Vector Machine,” Archive Des Science ISSN: 2231-5381 http://www.ijettjournal.org Page 110





