Non-Aldol Polyketide Syntheses Baran Group Meeting Hans Renata 09/01/2012
advertisement
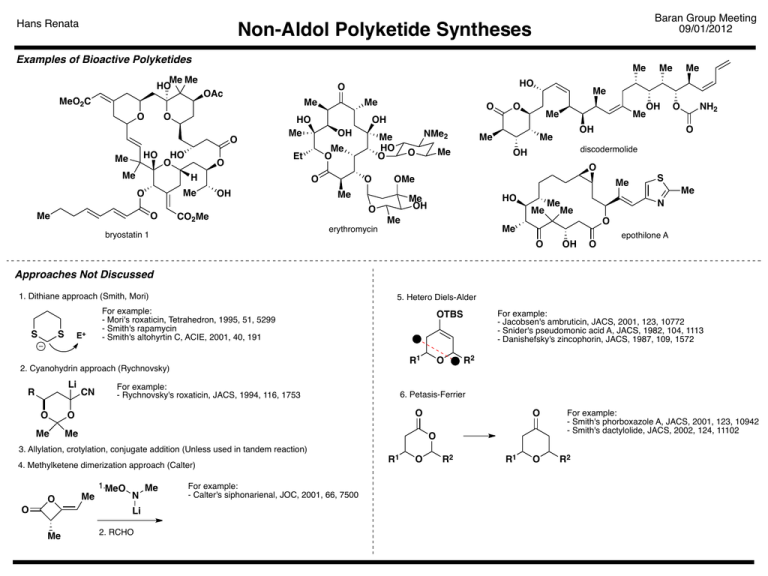
Hans Renata Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses Examples of Bioactive Polyketides Me Me HO MeO2C O Me Me O O HO Me HO O Me O HO Me Me Me O Me OH Me O Me Me O Me OH Me Me OH Me NH2 O O discodermolide OH O O OMe Me erythromycin Me HO Me OH O CO2Me bryostatin 1 NMe2 Me HO O Me O O OH O OH Et H O HO O OAc Me Me S Me N Me Me Me O Me epothilone A O OH O Approaches Not Discussed 1. Dithiane approach (Smith, Mori) S S E+ 5. Hetero Diels-Alder For example: - Mori's roxaticin, Tetrahedron, 1995, 51, 5299 - Smith's rapamycin - Smith's altohyrtin C, ACIE, 2001, 40, 191 R1 2. Cyanohydrin approach (Rychnovsky) Li R O O Me Me CN For example: - Jacobsen's ambruticin, JACS, 2001, 123, 10772 - Snider's pseudomonic acid A, JACS, 1982, 104, 1113 - Danishefsky's zincophorin, JACS, 1987, 109, 1572 OTBS For example: - Rychnovsky's roxaticin, JACS, 1994, 116, 1753 O R2 6. Petasis-Ferrier O O O For example: - Smith's phorboxazole A, JACS, 2001, 123, 10942 - Smith's dactylolide, JACS, 2002, 124, 11102 3. Allylation, crotylation, conjugate addition (Unless used in tandem reaction) 4. Methylketene dimerization approach (Calter) O O Me 1.MeO N Li Me 2. RCHO Me For example: - Calter's siphonarienal, JOC, 2001, 66, 7500 R1 O R2 R1 O R2 Hans Renata Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses Me Transition Metal Catalysis Me Me Me OH 1. Carbometalation Cp2Zr Cl Et Cl AlEt2 Cp2Zr Cl nBu AlEt2 Cp2Zr Cl Cl AlEt2 Et AlEt2 AlEt2 nOct ZrCl2 Me Me AlMe2 Ts N Et 40% 1. Zn(OTf)2 2. Pd vinylation OH Na/NH3 NH2 Me OMgCl Me OH OH nOct 2 Generation of Reduced Polypropionates (-)-ZACA CO2H borrelidin Et AlEt2 nOct H MgCl nOct O2 Et O Et EtMgBr 5% Cp2ZrCl2 Et/Decyl (major) Et3Al O fluvirucin B1 agylcon H nOct OH Me Peptide Coupling HN Al Et Me NC O Et nBu Me OH nBu nBu nOct Me Me OH OH Et3Al, cat. Cp2ZrCl2 Me OAc Me Magnesiation Cascade (Hoveyda) RCM AlEt2 nBu Cp2Zr Me 3. BH3 (10% from styrene, dr >80:1) Early observation (cf. Dzhemilev ethylmagnesiation and Kulinkovich reaction) - See ARKIVOC 2011, 34–53 for an extensive account nBu Me HO Zr-catalyzed Asymmetric Carboalumination (ZACA) Cp2ZrCl2 + Et3Al 1. Ac2O 2. RuCl3, NaIO4 NHTs 99% Et Me Me EtMgBr, 0.4% (S)-[EBTHI]-Zr-BINOL O Et n-PrMgBr, 3% Cp2TiCl2 Et Et BrMg ("~70 turnovers") BrMgO OH (Ph3P)2NiCl2, 72% Br Selected references: - Negishi et al. JACS, 1995, 117, 10771–10772 - Negishi et al. PNAS, 2004, 101, 5782–5787 - Negishi et al. JACS, 2005, 127, 2838–2839 Me Me Me Et Me OH OH (19% from styrene, dr >22/1.6/1) O Hoveyda et al. JACS, 1995, 117, 2943–2944 TPAP, NMO 65%, >99% ee Et OH Hans Renata Transition Metal Catalysis Asymmetric hydroformylation of dienes is possible Hydroformylation Me Catalyst-directing group on OH moiety (Breit) 1,2- Induction Me Me OH R CO O O Me Me Et O L*: PPh2 Me Rh H O H R R Me O (iso:n = 91:9, dr = 96:4) Me Proposed Stereochemistry Model O O Rh(CO)2acac, L*, 20 atm H2/CO (1:1) Et O Rh(CO)2acac, P(OPh)3, PPh2 20 bar H2/CO (1:1) O Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses O OH PPh2 PPh2 O P O Me O O O CO2H syn:anti > 9:1 Me Me Et Me ambruticin 1,3- Induction Jacobsen et al. JACS, 2001, 123, 10772–10773 Nozaki et al. Tetrahedron, 1997, 53, 7795–7804 Rh(CO)2acac, P(OPh)3, 20 bar H2/CO (1:1) Ph2P O Me O As part of tandem process... Ph2P Proposed Stereochemistry Model O O Rh P Ph2 O H Me anti:syn > 9:1 Me Me Si Me O O Me Me Me Me OH OH Me Me Me Me 59%, dr = 92:8 insertion/ RE Me CO O Si O Me Me Me Other accessible triads: OH Me O O OH [O] O O 80% Me H RhLn Ph O Si via: O Rh(CO)2acac, P(OPh)3, 20 bar H2/CO (1:1) O Me 1,2-Anti Induction Ph Rh cat., CO R R O Me Si Me Me R O O Ph facial approach Me dr > 99:1 O Selected references: - Liebigs Ann. Chem. 1997, 1841–1851 - Eur JOC, 1998, 1123–1134 - JOC, 2001, 66, 4870–4877 OH R (Start with alkyne, without oxidation step) OH O OH R (Start with alkyne, with oxidation step) Leighton et al. JACS, 1997, 119, 12416 Leighton et al. JACS, 2000, 122, 8587 Leighton et al. ACIE, 2001, 40, 2915 Hans Renata Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses Reductive Coupling Approach (See Reductive Coupling GM, IY, 2009) Use of silicon tether in reductive coupling (Phillips) Some Highlights iPr Construction of ene-1,5-diol motif (Micalizio) O " R1 Me O " Me OH R2 Me PMBO OH R1 Me iPr O R2 Me Me Me iPr ClTi(OiPr)3, iPrMgCl Si Me PMBO Me Me OH ClTi(OiPr)3 R'MgCl O R OiPr OiPr Me Ti O R'CHO Ti via: iPr O R R PMBO R' OH iPr O Si Me H Ti(OiPr)2 Me Me JACS, 2005, 127, 3694 HO iPrO OiPr OH R1 Ti OiPr Ti R4 R4 R3 OH R2 Applications in total synthesis: Me CoCl2, ligand, Me3Al, ethylene For example: JACS, 2010, 132, 3295 JACS, 2012, 134, 6556 Me OR Me NH Me R Me Me O O dictyostatin R MeO OMe O 1. Hydrovinylation R2 R3 JACS, 2006, 128, 2764 Me OH O Cobalt chemistry - untapped potential? R4 R1 R1 Me JACS, 2006, 128, 5340–5341 O R2 R3 Me Me Me Me If the third coupling partner is another alkyne: MeO Me Me via: OH Me 65% Me Me iPr O Si OH Me O H O O Me Me macbecin 1 O (ACIE, 2008, 47, 4005) Me O 2. Alder-ene reaction Me Me callystatin A (ACIE, 2008, 47, 7837) TMS EtO CoBr2, ligand, Zn, ZnI2 Me For example: OL, 2011, 13, 304–307 OL, 2011, 13, 5700–5703 Me MeO2C TMS Hans Renata Main Group Chemistry Silirane reactivity profile Acylsilane in Johnson's synthesis of zaragozic acid C O TBS MgBr O TBS O TBS tBuO2C tBuO2C OtBu TBSO BrMg O O OH H CO2tBu COtBu H tBuO2C tBuO2C OTBS OtBu O MgBr O MgBr O OtBu O TBSO tBuO O tBu tBu Si tBu tBu Bn tBu N OTMS tBu Si O CuI (20%) Si Me CO2H CO2H O Me 95% Et 1. Ph3PCH2Br 74% 2. PhMe2C(OOH), over 2 steps KH, CsF O OH Et O OH Et Me Me OH Me Me Me Me O tBu Si O SnBr4 Me OH H O HO2C tBu Et OAc Me then CuSO4, Ac2O Me Me tBu Me Me Me epi-stegobinone zaragozic acid C JOC, 2007, 72, 1027–1030 Variations on a theme tBu Si tBu R tBu tBu Si 1,2,4-triols from allenes tBu R Si tBu tBu Si AgLn R OTf electrophilic Ln = alkene, or phosphine ligand if present For mechanistic studies: JACS, 2003, 125, 10659–10663 (thermal) JACS, 2004, 126, 9993–10002 (Ag catalyzed) tBu Si R • tBu H Me Cy AgTFA; CuI, iPrCHO 70%, 96:3:1 dr 1. mCPBA 2. KOtBu TBAF tBu tBu AgX Acc. Chem. Res., 2000, 33, 813–820 O O electrophilic Metal-catalyzed R2 CHO Me Silirane ring opening (Woerpel) Thermal Si X R1 R2 O Johnson et al. JACS, 2008, 130, 17281–17283 Δ H 74% from butene AcO Me nucleophilic attack possible if X = leaving group tBu Si O [O] Bn Bn tBu R1 tBu X Si TBS Me 3 contiguous stereogenic centers in the correct oxidation state strategic protecting group scheme that masked every functional group except the 2º alcohol tBu Application in synthesis H OTBS 45–50% yield (15 g scale) diastereoselection >10:1 tBu O OtBu MgBr CO2tBu O TBSO tBuO2C Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses tBu Si O OH Me iPr iPr Me Cy 60% over 2 steps OH OH Cy OL, 2009, 11, 2173–2175 Hans Renata Lithiation-Borylation-Allylation Cascade (Aggarwal) Variations on a theme (contd.) Insertion into vinyl epoxides General idea: Si O tBu R2 tBu H O Si tBu R2 2% AgOTs R1 BR'2 Me Si 3 Li Highly diastereoselective O Me3Si functionalization possible R NiPr2 O 2 tBu O Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses R3 O tBu Si R1 tBu R H R O R Me OEt 37–80% Me3Si Proposed Zimmerman-Traxler-like TS tBu Si Me O R" SiMe3 Anti-diol RCHO OEt Me Me R Achieving stereocontrol tBu tBu Si O Me Me OH OH AgTFA R O R" tBu tBu H R" Si O Me3Si OCb OH H JACS, 2009, 131, 14182–14183 Reaction with dienoates H R3 H R1 BR'2 BR'2 OH B O B Si OH O O Me O tBu H Me R H C5H11 R R [O] O OH O R H OH R" SiMe3 R tBu O tBu OEt R" SiMe3 R" OH solandelactone E JACS, 2011, 133, 406–408 Reaction with dienes tBu Si tBu R1 tBu H 1% AgTFA R2 tBu H R1 Si OAc O tBu Si H tBu R3CHO H R2 R3 OAc Syn-diol R1 TBAF; Ac2O R1 H tBu Si O Δ R2 R3 R2 R3 Me3Si O B O O R B OH R O SiMe3 R" tBu [O] OH SiO2 H HO O tBu Si H tBu R" SiMe3 O R1 C5H11 R2 R3 JACS, 2012, 134, 12482–12484 OH H solandelactone F H O O OH R R" OH ACIE, 2010, 49, 4264–4268 ACIE, 2010, 49, 6673–6675 Org. Biomol. Chem. 2012, 10, 1795–1801 Hans Renata Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses Cyclic Stereocontrol - Pericyclic Reactions Use of Paterno-Buchi reaction (Asteltoxin, Schreiber) OBn Me O Me 63% O Me Me Me hν OBn O O Me mCPBA 80% OHMe ArCO2 OBn O O H HCl; Me2NNH2 72% OHMe EtMgBr O HO O O H O Me OH Me Et OBn O Me H OMe O O 88% OH Me2CO, CuSO4, CSA O Ph BuLi Me O asteltoxin H O S Ph S OH Et O Et OBn OH Me Me NNMe2 HO H OHMe OH O Me Me OH Me Et H O OBn OH Me O Et Me O Me Me O 55% over 2 steps Me O H Me O Me JACS, 1984, 106, 4186–4188 Directed nitrile oxide cycloaddition (Epothilone, Carreira) N O EtO P EtO 3 equiv EtMgBr OH OTIPS 3.3 equiv iPrOH + Cl Me OH Me 54%, 94% brsm O 2. LiCl, DBU EtO P EtO OTIPS N O OTIPS 1. TBSOTf N O N Me 70% (6:1 E:Z) Me OH Me S Me 1. SmI2, B(OH)3 2. BEt3, NaBH4 S OH Me 68% (9:1 E:Z) O Me N S Me Me Me Me O OH Me O OH epothilone A O O Me Me Me OH 1. SOCl2 2. TBAF N OH 77% OH OTIPS N Me S Me OH Me OH ACIE, 2001, 40, 2082 JACS, 2001, 123, 3611 JOC, 2001, 66, 6410 Hans Renata Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses Cyclic Stereocontrol - Pericyclic Reactions Cycloaddition-oxabicyclic ring opening (ionomycin, Lautens) O Me O Me X O base Me [H]; Protection O Me O Me Me OTIPS Ni(COD)2, (S)-BINAP, DIBAL-H OTIPS Me Me 95%, 93–95% ee "Redoxmassage" (D. Sarlah) Me 82% HO OTIPS Me 1. O3; NaBH4 2. DDQ PMBO 79% OH Me OH 23 OH O O 10 1 PMP 17 O O Me H Me H OH Me Me Me Me Me Me OH O Me O OTIPS OH ionomycin Note: C1–C10 fragment was synthesized via methylative oxabicyclic ring opening JACS, 1995, 117, 532 JACS, 1997, 119, 11090 OL, 2002, 4, 1879 Me Me C17–C23 fragment Pericyclic reaction with sulfur (methynolide, Vedejs) EtO2C Me Me S O Ac2O, AcOH; BF3.Et2O EtO2C 81% 1. K2CO3; then protect Me 2. DIBAL; MsCl, BuLi OAc Me 3. LiBEt H 3 Me S Me Me HO Me OH O Et Me O methynolide Me S Et H O Me OR Me Et OTf 76% dr= 16:1 CO2Et 59% S O O Me Me 35% tBu TfO OR H Me S H H H EtO2C S H Me OR Me Me Me OR Me Me H EtO2C 6 steps S OR Me Me H JACS, 1987, 109, 5878 JACS, 1989, 111, 8421, 8430 Hans Renata Baran Group Meeting 09/01/2012 Non-Aldol Polyketide Syntheses Conclusion POLY KETIDES "The armory of the synthetic chemist is richly endowed with weapons of great effectiveness in the stereoselective, or often stereospecific, generation of newly created asymmetric centers within rigid systems, as best exemplified by cyclic–and especially fused polycyclic–systems. By contrast, the construction of asymmetric arrays in a desired stereochemical sense in flexible, open chain systems is rare, or little understood when observed, and generalizations are dangerous." O Me Me Me Me Et Me O S H Me S Me NH O Me O MOMO O Me O Me MOMO OBn O Me CHO Me O Me Me "the use of sulfur atoms to create rings by bridging carbon atoms that are destined to become methyl groups; indeed, a glamorization of the lowly, usually modest methyl group, which at first sight would hardly be expected to play a prominent role in the direction of stereoselective synthetic operations!" Where are we today? Me Me halichomycin (TL, 1994, 35, 5013) H O Me H Me Me OH H O O O HO Me OMe O O OMe O Me O OMe Me Me OH OH neaumycin (OL, 2012, 14, 1254) O O O OH OH HN Me OH Me Me tripartilactam (OL, 2012, 14, 1258)