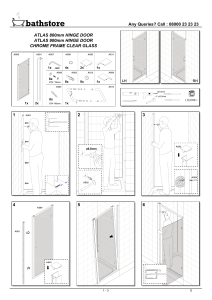
Advanced Spectroscopy Assignment
advertisement

Advanced Spectroscopy Assignment All questions must be attempted. AS02 – Mn in steel by UV/VIS 1. A S S E S S M E N T 2. 3. 4. Note: The subject webpage provides some background information for this method. AS03 – P in copper by UV/VIS 5. 6. What is the function of the molybdite and vanadate solutions? Explain why the more common colorimetric method for P known as the Molybdenum Blue method (because of the colour formed) would be unsuitable for this analysis. AS04 – Aspirin in tablets by UV 7. 8. Explain the reason for the samples being treated with NaOH solution and allowed to stand for 30 minutes. Explain why the samples are then acidified. AS05 – Benzoic acid in soft drinks 9. 10. T A S K Explain the reasons for the following preparation steps: a) addition of nitric acid b) boiling to remove oxides of nitrogen c) addition of ammonium persulfate d) addition of potassium periodate e) not adding periodate to the blank Explain why the standards were prepared using steels rather than from 1000 mg/L Mn standard. Compare the two calculation methods. Compare this method to that by AAS for Mn in steel. Explain the difference in results between the acidified and alkaline samples. Explain why this method would not be applicable to soft drinks such as Coca Cola and Fanta. AS08 – Zn in brass by AAS 11. 12. 13. Do you think that standard addition is necessary in this analysis? Would matrix-matched standards be a better approach? What would the ideal matrix-matched standard be made from? Is this sample a typical brass in terms of its zinc content? Justify your answer. AS09 – Analysis of xylene mixture by IR 14. 15. Explain the reasons for the choice of the analyte peaks. Compare this method with another technique capable of analysing this mixture for each component. AS11 – Na & K in cement by flame photometry 16. 17. 18. Give TWO reasons why mixed standards are superior to separate standards. Explain the function of the lithium. Explain why calcium is included in the standards. Submission date: Before the written exam for this subject