Metals 4
advertisement
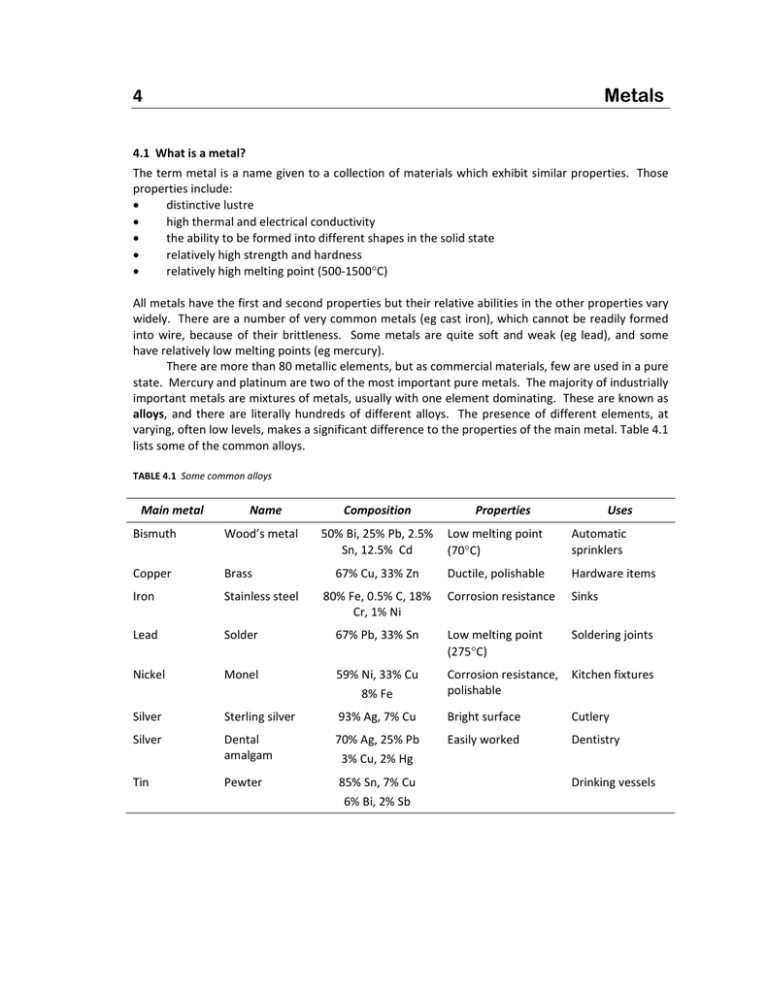
Metals 4 4.1 What is a metal? The term metal is a name given to a collection of materials which exhibit similar properties. Those properties include: distinctive lustre high thermal and electrical conductivity the ability to be formed into different shapes in the solid state relatively high strength and hardness relatively high melting point (500‐1500C) All metals have the first and second properties but their relative abilities in the other properties vary widely. There are a number of very common metals (eg cast iron), which cannot be readily formed into wire, because of their brittleness. Some metals are quite soft and weak (eg lead), and some have relatively low melting points (eg mercury). There are more than 80 metallic elements, but as commercial materials, few are used in a pure state. Mercury and platinum are two of the most important pure metals. The majority of industrially important metals are mixtures of metals, usually with one element dominating. These are known as alloys, and there are literally hundreds of different alloys. The presence of different elements, at varying, often low levels, makes a significant difference to the properties of the main metal. Table 4.1 lists some of the common alloys. TABLE 4.1 Some common alloys Main metal Name Bismuth Wood’s metal Copper Brass Iron Stainless steel Lead Solder Nickel Composition Properties 50% Bi, 25% Pb, 2.5% Low melting point Sn, 12.5% Cd (70C) Automatic sprinklers Ductile, polishable Hardware items Corrosion resistance Sinks 67% Pb, 33% Sn Low melting point (275C) Soldering joints Monel 59% Ni, 33% Cu 8% Fe Corrosion resistance, Kitchen fixtures polishable Silver Sterling silver 93% Ag, 7% Cu Bright surface Cutlery Silver Dental amalgam 70% Ag, 25% Pb 3% Cu, 2% Hg Easily worked Dentistry Tin Pewter 85% Sn, 7% Cu 6% Bi, 2% Sb Drinking vessels 67% Cu, 33% Zn Uses 80% Fe, 0.5% C, 18% Cr, 1% Ni 4. Metals 4.2 Metal refining With a few exceptions (eg gold, platinum), metals are found naturally as ores, which are rocks rich in one or two particular metallic elements. There will be a considerable amount of other mineral material of no use (known as gangue) mixed in with the valuable metallic minerals. This is often separated before any attempt to process the metal is made. The metals are present in an oxidised form, usually as oxide or sulfide salts. This is the stable form of the metal (as a salt), and to produce the metal in its elemental form requires lots of energy (by heat or electricity or both) and a reducing agent. This is known as the smelting process. Refining then takes the relatively impure metal and purifies it and adds other metals to produce the required alloy. Using iron as an example, it is mined as a mixture of haematite (Fe2O3), other iron minerals and worthless rock material. It is crushed, but no attempt is made to remove the gangue. At the iron smelter, it is mixed with coke (coal that has been heated in the absence of air to remove volatiles) and limestone in a blast furnace at temperatures reaching 1500C. Air, enriched with oxygen, is added. The coke (as carbon) is the reducing agent, either directly or CO, and these react with iron oxides to form liquid iron, as shown in Equation 4.1a and 4.1b. Most of the heat is produced by the exothermic reaction the coke forming CO. Fe2O3 (s) + C (s) Fe (l) + CO2 (g) Eqn 4.1a Fe2O3 (s) + CO (g) Fe (l) + CO2 (g) Eqn 4.1g The liquid accumulates at the base of the blast furnace, covered in a layer of molten slag, which is the gangue trapped with the limestone. Iron collected from the blast furnace is called pig iron. It is quite impure typically containing about 90% Fe, 5% C and 5% of other impurities such as Si, S and P. The high carbon content of pig iron makes it so brittle and hard that it is not really useful for anything. Therefore, the pig iron is taken to the refining stage, where it is treated with high pressure oxygen and more limestone. This brings the carbon to very small amount, and essentially removes the other elements. Now it can have controlled amounts of other elements added to it (even carbon) to produce the wide range of steels currently available. 4.3 Properties of metals As mentioned at the start of this chapter, metals are recognised by their properties, and used across a wide range of applications because of these properties. Physical properties are those which are inherent in the material, and do not rely on their interaction with other materials. These include density, melting point and conductivity. The so‐called mechanical properties – strength, hardness etc – are also physical properties, but are grouped together because they relate how the material behaves under some external force. Metals are used classified as ferrous or non‐ferrous, based on whether the main metal is iron or not. Ferrous alloys Metals based on iron are so widely used because of their good general metallic properties. It is also because iron ore is one of the more common ores, and the refining processes relatively simple. One property that iron does not perform well in is electrical conductivity, so you do not see used for that purpose. Its major use is based on its good compromise of strength, hardness and formability. Irons and steels are classified by their alloying elements, the most important of which is carbon. Steel is an iron alloy with less than 2% C. The basic grades of iron‐based metals are shown in Figure 4.1. Some of their uses are shown in Table 4.2. IPC 4.2 4. Metals TABLE 4.2 Uses of iron alloys Alloy Applications Cast irons Readily castable, strong; used in fence posts Mild steels quite plastic, used in nails, chains, cables Medium steels tougher than mild steel; used in building components, such as girders High carbon steels much harder; used in tools and springs Alloy steels other elements added to produce a wide range of steels, such as chromium and nickel in stainless steel IRON ALLOYS CAST IRONS 2-3.8%C ALLOY STEELS STEELS <2%C FIGURE 4.1 Classes of irons and steels Mild (<0.2%C) CARBON STEELS Medium (0.2-0.6%C) High (0.6-1.5%C) Non‐ferrous alloys The two most important metals other than iron in metals are copper and aluminium. However, at least 20 other metals are used in industrially because of their particular properties. The features of non‐ferrous alloys that make them better than any ferrous metal include: density thermal conductivity electrical conductivity heat resistance colour corrosion resistance formability strength IPC 4.3 4. Metals EXERCISE 4.1 Suggest non‐ferrous metals which display the following features. Density Low High Thermal conductivity Low High Electrical conductivity Low High Heat resistance High Colour Different Corrosion resistance High Formability High Strength High 4.4 Heat treatment of metals After solidifying, many metals go through further heating steps to modify their properties. This does not involve remelting them. Iron is one metal that is almost always heat treated. You would probably expect that a metal, be it pure or an alloy, is a completely homogenous material. In fact, this is not the case. The carbon that is trapped in, or added to iron and steel, is soluble to some extent, but as the iron cools, then solidifies, it begins to precipitate (as graphite) and/or form a hard, brittle carbide compound with the iron (cementite, Fe3C). As well, the iron as it solidifies and crystallises, produces two distinct forms – austenite (soft and non‐magnetic) and ferrite (soft and magnetic) – which differ in atomic arrangement, and therefore, physical properties, Pearlite can also form – it is a structure formed by layering of ferrite and cementite. Altogether this produces a very complex and heterogenous mixture. Figures 4.2 and 4.3 are known as phase diagrams, and show the relationship between %C, temperature and physical/chemical state. Phase diagrams are important because they help in understanding why heat treatment actually achieves something. How do you read what a phase diagram is telling you? Firstly, it tells you information about iron of a particular composition, eg 4.3% C. Secondly, it tells you what happens to that material as it cools very slowly. EXAMPLE What happens to a steel containing 4.3%C as it cools very slowly from liquid? The steel will remain completely molten until about 1131C (known as the upper critical temperature). Then it is will solidify into a mixture of austenite and cementite. Once it cools to below 723C (the lower critical temperature), the austenite will reform into ferrite. You will notice that the boundary between liquid and part liquid‐part solid is not uniform across different %C, and dips to a low point at 4.3%C at 1131C. This pronounced dip is known as a eutectic point. There are a number of other of these on this diagram. IPC 4.4 4. Metals FIGURE 4.2 Phase diagram for iron/carbon FIGURE 4.3 More detail in Fe/C phase diagram for steels IPC 4.5 4. Metals In the heat treatment of metals, the cooling process that is implied by the phase diagram is often interrupted by a sudden cooling, which “freezes” the metal in the form that it had reached when the cooling was applied. It does not have the opportunity to reform as it would otherwise to do if cooling slowly. EXERCISE 4.2 (a) Describe what happens to a 1% C steel as it cools. (b) What would happen to a 0.5% steel if it was heated slowly from 25C back to 1000C, and then allowed to cool back to 750C before being suddenly chilled back to 25C? What You Need To Be Able To Do IPC define important terminology name common alloys and give their composition (not % values) name common alloying elements in steel and indicate their effect on properties list important properties of non‐ferrous metals interpret a phase diagram describe heat treatment processes 4.6



