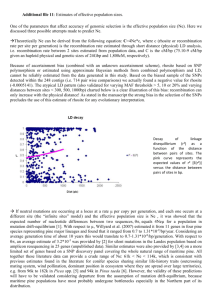
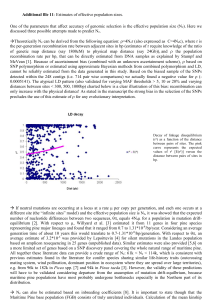
Interspecific phylogenetic analysis enhances intraspecific phylogeographical inference: a case study in
advertisement

Molecular Ecology (2007) 16, 3926– 3937 doi: 10.1111/j.1365-294X.2007.03461.x Interspecific phylogenetic analysis enhances intraspecific phylogeographical inference: a case study in Pinus lambertiana Blackwell Publishing Ltd A A R O N L I S T O N ,* M A R I A H P A R K E R - D E F E N I K S ,* J O H N V . S Y R I N G ,*‡ A N N W I L L Y A R D ,*§ and R I C H A R D C R O N N † *Department of Botany and Plant Pathology, 2082 Cordley Hall, Oregon State University, Corvallis, Oregon 97331, USA, †Pacific Northwest Research Station, USDA Forest Service, 3200 SW Jefferson Way, Corvallis, Oregon 97331, USA Abstract Pinus lambertiana (sugar pine) is an economically and ecologically important conifer with a 1600-km latitudinal range extending from Oregon, USA, to northern Baja California, Mexico. Like all North American white pines (subsect. Strobus), sugar pine is highly susceptible to white pine blister rust, a disease caused by the fungus Cronartium ribicola. We conducted a chloroplast DNA (cpDNA) survey of Pinus subsect. Strobus with com­ prehensive geographical sampling of P. lambertiana. Sequence analysis of 12 sugar pine individuals revealed strong geographical differentiation for two chloroplast haplotypes. A diagnostic restriction site survey of an additional 72 individuals demarcated a narrow 150-km contact zone in northeastern California. In the contact zone, maternal (megagame­ tophtye) and paternal (embryo) haplotypes were identified in 31 single seeds, demonstrating bidirectional pollen flow extending beyond the range of maternal haplotypes. The frequencies of the Cr1 allele for white pine blister rust major gene resistance, previously determined for 41 seed zones, differ significantly among seed zones that are fixed for the alternate haplotypes, or contain a mixture of both haplotypes. Interspecific phylogenetic analysis reveals that the northern sugar pine haplotype belongs to a clade that includes Pinus albicaulis (whitebark pine) and all of the East Asian white pines. Furthermore, there is little cpDNA divergence between northern sugar pine and whitebark pine (dS = 0.00058). These results are consistent with a Pleistocene migration of whitebark pine into North America and subsequent chloroplast introgression from whitebark pine to sugar pine. This study demonstrates the importance of placing phylogeographical results in a broader phylogenetic context. Keywords: chloroplast introgression, Cronartium ribicola, phylogeography, Pinus lambertiana, Pinus subsect. Strobus, white pine blister rust resistance Received 13 January 2007; revision received 17 April 2007; accepted 11 June 2007 Introduction Pinus has been described as ‘the most economically and ecologically significant tree genus in the world’ (Richardson & Rundel 1998). Support for this claim is found in the large Correspondence: A. Liston, Fax: 1 541 737 3573. E-mail: listona@science.oregonstate.edu §Present address: Department of Biology, University of South Dakota, Vermillion, SD 57069, USA ‡Present address: Department of Biological and Physical Sciences, Montana State University–Billings, Billings, MT 59101, USA number of population genetic studies conducted in pine species. Ledig (1998) summarized genic diversity statistics (primarily from isozymes) for 51 of the c. 110 species of pine. Over the last 15 years, at least 26 species of pine have been evaluated for among-population DNA variation (tabulated in Petit et al. 2005; see also Chiang et al. 2006; Navascues et al. 2006). The focus of many of these studies is phylogeographical inference using chloroplast DNA (cpDNA). Despite the prevalence of interspecific hybridization in pines (Ledig 1998), few of these studies sample other related species, and thus cannot place the within-species genetic variation in a broader phylogenetic © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd P H Y L O G E N Y A N D P H Y L O G E O G R A P H Y 3927 context. This study of Pinus lambertiana, sugar pine, demonstrates how resolution of interspecific phylogeny can have a profound impact on the interpretation of intraspecific phylogeographical results. Just as studies can be enhanced by ‘putting the geography into phylogeography’ (Kidd & Ritchie 2006), it is imperative to incorporate a broad phylogenetic perspective as well. Pinus lambertiana is one of the c. 20 species of subsection Strobus (Gernandt et al. 2005; Syring et al. 2007). This clade is known by the common name ‘white pines’ and is distributed discontinuously throughout the Northern Hemisphere (Table 1). The monophyly of subsect. Strobus is strongly supported by chloroplast sequences (Gernandt et al. 2005; Eckert & Hall 2006) and nuclear ribosomal DNA (Liston et al. 1999) and moderately supported by a low copy nuclear locus (Syring et al. 2007). The 20 species share a similar vegetative morphology (five relatively narrow and strongly amphistomatic needles per fascicle) but differ dramatically in ovulate cone size (from 5 cm in Pinus pumila to 60 cm in P. lambertiana) and shape. The five Pinus species (P. albicaulis, P. cembra, P. koraiensis, P. pumila, P. sibirica) with indehiscent ‘closed’ cones adapted for bird dispersal were traditionally treated as ‘subsect. Cembrae’, or stone pines. Like all North American members of subsect. Strobus, sugar pine is highly susceptible to white pine blister rust, a disease caused by the heterocyclic rust fungus Cronartium ribicola. This pathogen is native to Asia (Kinloch 2003) and was accidentally introduced to North America in the eastern United States and British Columbia in the early 20th century (Mielke 1943; Scharpf 1993). It has subsequently spread to all species of subsect. Strobus that occur in the United States and Canada (Scharpf 1993; Kinloch 2003). The disease results in cankers that girdle the main stem and kill infected seedlings and trees (Kinloch & Scheuner 2004). While a mapped locus (Cr1) that confers qualitative (major gene) resistance has been identified in sugar pine (Kinloch 1992, 2003; Devey et al. 1995), its frequency in populations is typically low. Cr1 frequency varies from less than 10% in the southern part of the range of P. lambertiana to near absence in the north (Kinloch 1992; summarized in our Table 2). Attempts to increase the frequency of white pine blister rust resistance have prompted federal (USDA Forest Service, US Bureau of Land Management) and private agencies to make extensive seed collections for this species, and to initiate large-scale screening programmes. We used DNA sequences of two chloroplast loci to conduct a phylogenetic analysis of North American and Eurasian members of Pinus subsect. Strobus, in concert with a phylogeographical survey of P. lambertiana. Our study documents significant cpDNA divergence between two major haplotypes in P. lambertiana. The distribution of these haplotypes is concordant with the geographical distribution of white pine blister rust major gene resistance. A narrow contact zone between haplotypes, and limited © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd divergence within each haplotype, strongly suggests that secondary contact between two cytoplasmically divergent groups has occurred in the (evolutionarily) recent past. Our phylogenetic analysis provides evidence that the northern populations of P. lambertiana may have obtained their chloroplast via introgression from P. albicaulis, whitebark pine. The integration of phylogenetic and phyl­ ogeographical approaches allowed us to recover this unex­ pected evolutionary history. Materials and methods Organismal sampling and DNA genotyping Pinus lambertiana Douglas is an economically and eco­ logically important conifer with a 1600-km latitudinal range extending from Oregon, USA to northern Baja California, Mexico. Eighty-four individuals representing the geographical range of this species were included in this analysis. Nineteen additional species from Pinus subsect. Strobus were sampled for the phylogenetic analysis (Table 1), and Pinus gerardiana from subsect. Gerardianae was used as the outgroup. DNA was extracted from the haploid megagametophyte of individual seeds as described in Syring et al. (2007). Polymerase chain reaction (PCR) amplification followed Gernandt et al. (2005). Approximately 90% of the chloroplast matK open reading frame (1404 bp) and c. 150 bp of the 3′ trnK (UUU) intron (see Hausner et al. 2006 for a recent review) were amplified using primers matK1F (Wang et al. 1999) and ORF515–900F (Gadek et al. 2000). The chloroplast trnG (UCC) intron (c. 780 bp) was amplified using the primers 3′ trnG and 5′ trnG2G (Shaw et al. 2005). For divergence time estimates between Pinus albicaulis and P. lambertiana (described below), three additional loci were added to the cpDNA data set for these species. These include new sequences for the trnL-trnF intergenic region (including trnL exon 1 and its intron) and the rpl16 intron (Shaw et al. 2005), as well as previously published rbcL sequences (Gernandt et al. 2005). Predicted amplicon lengths were based on the Pinus koraiensis chloroplast genome (EW Noh, JS Lee, YI Choi, MS Han, YS Yi, and SU Han, unpublished, AY228468). Uncloned PCR products were submitted to High-Throughput Sequencing Solutions (University of Washington) for ExoSAP purification and automated capillary sequencing. Electropherograms were examined and aligned with bioedit 7.0.5.2 (Hall 1999). All new sequences are deposited in GenBank under the Accessions nos EF546699–EF546759. Chloroplast matK and trnG sequences from 12 individuals of P. lambertiana identified two divergent haplotypes (see results) abbreviated N (for North) and S (for South). Using methods described in Liston (1992), we screened 72 individuals for an AluI restriction site that differentiates these two haplotypes. This assay is diagnostic for a C/T 3928 A . L I S T O N E T A L . Table 1 Geographical origin and GenBank Accessions for samples sequenced for the cpDNA matK and trnG intron loci Pinus species albicaulis* albicaulis* albicaulis* albicaulis* albicaulis*‡ albicaulis* armandii† armandii† ayacahuite ayacahuite ayacahuite bhutanica cembra cembra cembra chiapensis chiapensis chiapensis dalatensis† flexilis flexilis flexilis koraiensis koraiensis koraiensis kwangtungensis lambertiana* lambertiana lambertiana* lambertiana*‡ lambertiana* lambertiana lambertiana lambertiana lambertiana*‡ lambertiana lambertiana lambertiana monticola monticola monticola morrisonicola parviflora parviflora peuce pumila pumila pumila sibirica sibirica strobiformis strobiformis strobiformis strobus strobus strobus wallichiana† wallichiana gerardiana† Haplotype a b a b c a b c N N N N N N S S S S S S′ a b b a b a b b a b Country Administrative unit matK accession trnG accession USA USA USA USA USA USA China Taiwan Honduras Mexico Mexico India Austria Switzerland Romania Guatemala Mexico Mexico Vietnam USA USA Canada Russia Japan South Korea China USA USA USA USA USA USA USA USA USA USA USA Mexico USA Canada USA Taiwan Japan Japan Bulgaria Japan Japan unknown Russia Russia Mexico Mexico USA USA Canada USA Pakistan Nepal Pakistan California: Mono Co. California: Siskiyou Co. Montana Oregon Washington Wyoming Anhui Kaohsiung La Paz Mexico Michoacan West Kameng EF546699 ″ ″ ″ ″ ″ EF546700 EF546701 EF546702 ″ ″ EF546703 EF546704 EF546705 EF546706 EF546707 ″ ″ EF546708 EF546709 EF546710 EF546711 EF546712 ″ ″ EF546713 EF546715 ″ ″ ″ ″ ″ EF546714 ″ ″ ″ ″ EF546716 EF546717 EF546718 ″ EF546719 EF546720 ″ EF546721 EF546722 ″ ″ EF546723 EF546724 EF546725 EF546726 ″ EF546727 ″ ″ EF546728 EF546729 AY115801 EF546730 ″ ″ ″ ″ ″ EF546731 ″ EF546732 ″ ″ EF546733 EF546734 ″ ″ EF546735 ″ ″ EF546736 EF546737 ″ ″ EF546738 ″ ″ EF546739 EF546741 ″ ″ ″ ″ ″ EF546740 ″ ″ ″ ″ ″ EF546742 EF546743 ″ EF546744 EF546745 ″ EF546746 EF546747 ″ ″ EF546748 ″ EF546749 ″ ″ EF546750 ″ ″ EF546751 ″ EF546752 Chiapas Guerrero Kon Tum California Colorado Alberta California: seed zone 091 California: seed zone 372 California: seed zone 516 California: seed zone 732 Oregon: seed zone 472 Oregon: seed zone 731 California: seed zone 120 California: seed zone 526 California: seed zone 731 California: seed zone 992 California: seed zone 994 Baja California Oregon British Columbia California Hokkaido Honshu Hokkaido Hokkaido Krasnoyarsk Krai Kemorovo Coahuila Durango Texas Minnesota Newfoundland North Carolina Punjab Karnali Gilgit Haplotypes N, S and S’ for P. lambertiana are described in the text. In other species, the letters a, b and c were applied as needed for multiple haplotypes within a species. Voucher specimens are deposited at Oregon State University (OSC), unless indicated otherwise; *sequenced for trnL-F, GenBank Accession nos EF546753 –EF546756; †Voucher deposited at the Silva Tarouca Research Institute for Landscape and Ornamental Gardening, Prùhonice, Czech Republic (RILOG); ‡ sequenced for rpl16, GenBank Accession nos EF546757–EF546759. © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd P H Y L O G E N Y A N D P H Y L O G E O G R A P H Y 3929 Table 2 The frequency of the white pine blister rust resistance gene Cr1 in sugar pine seed zones, compared to the number of southern and northern haplotypes. Data in columns 2 and 3 are from the 41 seed zones sampled by Kinloch (1992) Seed zone Trees/seeds sampled Frequency of major gene resistance Oregon: coast ranges and Cascades 090 10/74 0.0000 452 472 491 24/430 0.0000 492 7/231 0.0000 501 8/237 0.0127 502 18/379 0.0053 511 12/204 0.0049 512 9/205 0.0000 681 701 702 703 8/157 0.0000 721 Northwest California: coast ranges 081 2/15 0.0000 091 36/1763 0.0085 095 8/136 0.00 301 162/4752 0.0061 302 5/137 0.00 303 311 28/683 0.0073 321 141/5197 0.0073 322 5/302 0.00 331 3/44 0.00 332 340 33/2030 0.0030 371 4/190 0.0053 372 101/4065 0.0096 Northeast California: Cascades 516 8/265 0.00 521 12/779 0.0026 522 264/15815 0.0192 523 42/1869 0.0316 731 732 7/174 0.0632 741 9/538 0.00 742 771 Eastern California: Sierra Nevada 524 85/3141 0.0274 525 116/5099 0.0322 526 222/7714 0.0460 531 91/2976 0.0339 532 16/941 0.0659 533 16/635 0.0819 534 124/2668 0.0727 540 44/1077 0.0706 772 14/222 0.0180 California: central coast range 120 93/2133 0.0886 Southern California: transverse ranges 992 8/644 0.0699 993 29/463 0.0324 994 31/337 0.0297 997 32/517 0.0561 Baja California: Sierra San Pedro Martír 12/254 0.00 © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd Haplotype group N N N N N N N N N N N N N N N haplotype S haplotype Frequency of S haplotype 1 1 1 0.00 0.00 0.00 1 1 1 1 1 0.00 0.00 0.00 0.00 0.00 1 0.00 N N N N N N N N N N N N N N 2 2 0.00 0.00 1 1 1 1 1 1 1 1 1 1 1 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 0.00 N N Mixed Mixed Mixed Mixed N N Mixed 1 3 4 1 1 8 2 4 1 2 0.00 0.00 0.43 0.75 0.67 0.33 0.00 0.00 0.67 S S S S S S S S S 3 2 2 1 1 1 1 1 3 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 1.00 S 1 1.00 S S S S 1 1 1 1 1.00 1.00 1.00 1.00 S 1 3 3 2 4 1.00 3930 A . L I S T O N E T A L . polymorphism at position 1352 in matK. Since chloroplasts are paternally inherited in Pinus (Neale & Sederoff 1989), the AluI polymorphism was also used to determine mater­ nal vs. pollen cpDNA haplotype by extracting DNA from megagametophyte (maternal) and embryo (paternal) tissue in 31 individual seeds. nonparametric bootstraps were performed using poptools version 2.7 (Hood 2006). For mapping, the program trs2ll (Wefald 2001) was used to convert township/range/section localities to latitude and longitude. Phylogenetic, phylogeographical and statistical analyses Phylogeographical haplotype variation in sugar pine Phylogenetic analyses and constraint tests were conducted with paup* 4.0b10 (Swofford 2002) following the procedures outlined in Syring et al. (2005). Indels were added to the parsimony data matrix as binary characters. Parsimony analysis was conducted with a heuristic search strategy of 1000 random addition sequences and tree-bisection– reconnection swapping. Branch support was assessed using 2000 bootstrap replicates. Indels and duplicate sequences were excluded, and the most appropriate likelihood model was selected using the method of Posada & Crandall (1998) and AIC scores at the findmodel website (http://hcv.lanl.gov/content/hcv-db/findmodel/ findmodel.html). To evaluate whether sequences diverged at clock-like rates, maximum-likelihood trees were estimated using the GTR + γ model as implemented in paup* 4.0b10 (Swofford 2002). Likelihood scores obtained with and without a molecular clock constraint were evaluated using the likelihood ratio test (LRT) of Muse & Weir (1992). Under assumptions of a molecular clock, the divergence time (Tdiv) between two groups of sequences is approximately Tdiv = dS/2µ, where dS is the average pairwise distance among sequences at presumably neutral (synonymous and noncoding) sites and µ is the neutral mutation rate. Estimates of dS were calculated with dnasp 4.10.9 (Rozas et al. 2003; note that the program uses the abbreviation Ks). The estimate of µ for Pinus cpDNA (0.22 × 10–9 silent substitutions site–1 years–1; standard error = 0.55 × 10–10) is based on a divergence time of 85 million years between the two subgenera of Pinus; see Willyard et al. (2007) for details. Divergence times estimated here are reported with ± one standard error. To investigate whether the white pine blister rust major gene resistance allele (Cr1) frequencies were different among chloroplast haplotype classes, 41 tree seed zones used in blister rust screening (Table 2; Kinloch 1992) were classified as either fixed for the N haplotype, fixed for the S haplotype, or polymorphic for the two haplotypes. Oneway analysis of variance (anova) was used to examine variance partitioning and to test the hypothesis that means were equivalent among these three groups. Given the large number of zeros present in estimated Cr1 allele frequencies (especially in the northern part of the range), we also esti­ mated means and confidence intervals for allele frequencies in the N, S, and mixed haplotype groups using 10 000 nonparametric bootstrap resamplings. Statistical and Sequences of cpDNA matK and trnG intron in 12 Pinus lambertiana individuals revealed two predominant haplotypes with fixed differences at 10 sites (seven in matK, including three amino acid replacements; three in the trnG intron). One additional haplotype was observed in the Baja California individual (an autapomorphic matK replacement substitution). The sequence results combined with AluI restriction digest assays for 72 individuals demonstrated an abrupt transition in the distribution of the two predominant haplotypes (Fig. 1A). Plants from Oregon and northwestern California (Klamath mountains and North Coast range) were fixed for a common haplotype ‘N’ (thymine at position 1352 of matK), while plants from the Sierra Nevada and Transverse and Peninsular ranges in California were fixed for the alternate haplotype ‘S’ (cytosine at position 1352). The contact zone between the N and S haplotypes occurs near latitude 40°30′N in northeastern California, and the zone of polymorphism is remarkably well-defined, spanning less than 150 km of the c. 1600-km latitudinal range of this species (Figs 1 and 2). Megagametophyte and embryo comparisons in 31 individuals from the contact zone revealed that 12 (39%) seeds contained different maternal and paternal haplotypes, indicating that seedlings are frequently sired by pollen parents with different haplotypes than the ovulate parent. Embryos with the S haplotype were found in seeds from eight N haplotype maternal trees distributed in seed zones 522, 523, 732 and 771 (Fig. 2). The converse pattern was also observed as four S haplotype maternal plants from seed zones 522 and 731 produced seed containing N haplotype embryos (Fig. 2). At least one sugar pine individual was assayed for the S vs. N haplotype in 35 of the 41 seed zones sampled by Kinloch (1992) in his survey of Cr1 allele frequencies (the factor conferring major gene resistance to white pine blister rust; Table 2), with intensive sampling in the contact zone (Fig. 2). The haplotype for six unassayed seed zones (one in northwestern California and five in Oregon) was inferred to be N based on results from adjacent seed zones. The Baja California haplotype S′ (differing from S by one substitution, Fig. 3) was grouped with S for this analysis. The N, S, and polymorphic seed zones showed significantly different Cr1 frequencies by one-way anova (F = 32.3, P = 7.6 × 10 – 9). The N haplotype seed zones had the lowest Cr1 allele frequency (0.0032 ± 0.0038; n = 23), mixed Results © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd P H Y L O G E N Y A N D P H Y L O G E O G R A P H Y 3931 Fig. 1 (A) Sampled individuals of Pinus lambertiana in western North America. Yellow squares represent the S haplotype and blue squares represent the N haplotype. Forest tree seed zones sampled by Kinloch (1992) and/or this study are shaded according to the frequency of the Cr1 allele (Table 2), ranging from 0% (no shading) to 8.9% (dark grey, seed zone 120). Seed zones outlined in red were not sampled for Cr1. The haplotype contact zone (green rectangle) is shown in more detail in Fig. 2. (B) Approximate geographical distribution of P. lambertiana (dark grey) and P. albicaulis (light grey) from Critchfield & Little (1966). Red triangles represent P. albicaulis samples used in this study. haplotype seed zones had intermediate Cr1 frequencies (0.0380 ± 0.0185; n = 3), and S haplotype seed zones had the highest Cr1 frequencies (0.0484 ± 0.0260; n = 15). Nonparametric bootstrapping resulted in the same mean allele frequencies and confidence intervals. Phylogenetic relationships and variation in sugar pine and other white pine species. Alignment of matK and trnG intron sequences required one 6-bp indel in the matK ORF and four indels (1, 4, 10 and 15 bp) in the trnG intron. Three of the indels were confined to Pinus parviflora, the 15-bp trnG intron indel was shared by P. ayacahuite, P. flexilis, P. strobiformis and P. peuce, and the 1-bp indel was autapomorphic in P. armandii ‘b’. Combined phylogenetic analysis of matK (1545 bp), trnG intron (746 bp) and the five indels resulted in two most parsimonious trees of length 57 with a consistency index of 0.91 (Fig. 3). The two trees differ in the resolution of Pinus monticola as a grade © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd (shown) or clade (not shown). All Asian species of subsect. Strobus form a strongly supported clade that includes the North American Pinus albicaulis and the P. lambertiana N haplotype. At these two loci, no sequence divergence was found among northern P. lambertiana, P. albicaulis and representatives of six Asian Pinus species (P. dalatensis, P. koraiensis, P. pumila, P. sibirica, P. wallichiana, P. kwan­ gtungensis) and P. cembra from Romania. The P. lambertiana S haplotypes and the remaining North American white pine species form a grade, with P. monticola in a strongly supported sister position to the Eurasian clade. Constraining P. lambertiana samples to monophyly results in trees of 66 steps, which is significantly longer based on both Templeton (P = 0.0039) and Kishino –Hasegawa tests (P = 0.0027). Maximum-likelihood trees obtained with and without a molecular clock were topologically identical to one of the most parsimonious trees and were not significantly 3932 A . L I S T O N E T A L . Fig. 2 Detail of the contact zone between the S (yellow) and N (blue) cpDNA haplotypes of Pinus lambertiana. Squares represent the maternal (megagametophyte) haplotype and circles represent the pater­ nal (embryo) haplotype. Asterisks denote white pine blister rust resistant individuals. different from each other based on the LRT (∆ln L = 10.44013, χ2 = 20.88, d.f. = 19, P = 0.34). This suggests that the sequences are diverging at equivalent rates, and a simple molecular clock calculation can be applied. The mean dS between P. monticola and the Asian clade is 0.00232, resulting in an estimated divergence of 5.3 ± 1.3 million years ago (Ma; Late Miocene–Early Pliocene). Sequences of 5799 bp of cpDNA (adding rbcL, rpl16 and trnL-F to the matK and trnG used in the phylogenetic analysis) revealed a single substitution in trnL-F between a P. albicaulis accession (Washington state) and an N haplotype P. lambertiana (dS = 0.00058), resulting in an estimated divergence of 0.6 ± 0.15 Ma (Pleistocene). Sequences of trnL-F from an additional five accessions of P. albicaulis and three of N haplotype P. lambertiana (Table 1) confirmed that this is a fixed difference between these two taxa. The estimated divergence time between the two P. lambertiana chloroplast haplotypes is 15.5 ± 3.9 Ma (dS = 0.00681) and between the S haplotype of P. lambertiana and the other North American white pines is 9.0 ± 2.25 Ma (dS = 0.00396). Discussion The pattern of genetic subdivision in sugar pine Previous accounts of sugar pine have described neither morphological nor ecological differences that can be associated with the phylogeographical pattern observed here. In fact, Mirov (1967) described Pinus lambertiana as ‘rather stable morphologically’ (p. 142) and he considered it to be a prime example of a ‘good species’ that is ‘clearly delimited [and] can be identified without difficulty’ (p. 531). The southern limit of the contact zone (Fig. 1A) does coincide with the interface of the Cascade and Sierra © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd P H Y L O G E N Y A N D P H Y L O G E O G R A P H Y 3933 Fig. 3 One of two most parsimonious trees estimated from cpDNA matK and trnG intron sequences. Bootstrap values are shown below the branches. Tree length = 57, consistency index = 0.91, retention index = 0.97. When applicable, haplotypes (see Table 2) and the number of individuals that share a particular sequence and haplotypes follow the species names. Nevada mountain ranges, characterized by relatively recent volcanic activities and predominantly metamorphics (with granitic intrusions and volcanic activities), respectively (Hickman 1993). Although this geological transition is used to demarcate two floristic subregions, there is no apparent vegetational break between the forests of the Cascades and northern Sierra Nevada (Hickman 1993). In her PhD thesis, Martinson (1997) conducted an analysis of allozyme data collected by Conkle (1996, abstract only). Forty populations and 400 individuals of P. lambertiana were assayed at 30 allozyme loci. Average heterozygosity was 0.22, and no geographical region showed reduced genetic diversity. Clustering of genetic distances separated populations from Oregon and northwestern California from populations in the Sierra Nevada. Unfortunately, no populations were sampled in the chloroplast haplotype contact zone in northeastern California. Thus, although the reported allozyme differentiation may correspond to the chloroplast haplotype distribution, it will require allozyme sampling in the contact zone to confirm this. © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd The allozyme data also separated the Sierra Nevada populations from those sampled in southern California and Baja California. This division is not apparent in our data set. However, a unique substitution was found in the matK sequence of the Baja California individual. This evidence for genetic isolation is consistent with the geographical isolation of this disjunct population (Fig. 1A,B). The narrow transition zone between the N and S haplo­ types of P. lambertiana suggests that these populations have only recently come into contact. This would be consistent with a Holocene range expansion from separate northern and southern refugia. There is abundant evidence from the pollen record for postglacial movement of pines (Mohr et al. 2000; Thompson & Anderson 2000). Unfortunately, individual Pinus species cannot be identified from pollen. Narrow haplotype (chloroplast or mitochondrial) transition zones observed in other western North American plants (Soltis et al. 1997; Aagaard et al. 1998; Latta & Mitton 1999; Johansen & Latta 2003) have also been attributed to postglacial contact of previously separated populations. The examination of maternal and paternal haplotypes offers insight into the dynamics of dispersal at the contact zone (Fig. 2). The two P. lambertiana trees sampled in the southern part of seed zone 731 represent a population that is isolated on Happy Camp mountain, Modoc County. These trees have the S haplotype, and are presumed to have colonized this location by long-distance seed dispersal. The closest sampled potential source is c. 90 km away. The large (228 ± 40 mg) and ‘flimsy’ winged seeds of P. lambertiana are ‘seldom dispersed far by wind’, but caching by Steller’s jays (Thayer & Vander Wall 2005) and Clark’s nutcrackers (D. Tomback, personal communication) can potentially lead to long-distance dispersal. No other example of a disjunct haplotype was observed. Embryos possessing the S haplotype occur up to 25 km north of the northern­ most potential source trees, indicating that pollen flow advances ahead of seed dispersal. The two Happy Camp mountain trees whose megagametophytes carry the S haplotype have apparently been pollinated by trees of the N haplotype. Comparison of chloroplast and mitochondrial haplotypes in a Pinus ponderosa contact zone in western Montana has found a similar pattern of more extensive pollen flow and rare long-distance seed dispersal (Latta & Mitton 1999; Johansen & Latta 2003). One of the most surprising results of our study is the concordance between the distribution of the two cpDNA haplotypes and the relative frequency of a white pine blister rust major gene resistance locus (Cr1) in sugar pine. Kinloch (1992) described the pattern of Cr1 frequency as a cline. In contrast, the significant differences in Cr1 frequency observed among the three haplotype groups (N, S, and mixed) suggests that the gene frequency does not change in a gradual manner, but rather shows the same abrupt transition as observed in the chloroplast. There is no 3934 A . L I S T O N E T A L . evidence for a causal link between the cpDNA haplotype and Cr1 distribution patterns. It is well-established that resistance shows nuclear inheritance (Kinloch 1992), and the highest frequencies of Cr1 (4 – 9% per seed zone) are far lower than the frequency of the S haplotype. Furthermore, examination of three resistant trees (heterozygous for the dominant Cr1 allele; J. Gleason, unpublished data) in the contact zone found both N and S haplotypes (Fig. 2). Note that all other genotyped individuals in the contact zone are nonresistant ( J. Gleason, unpublished data). We predict that the concordance between the Cr1 and chloroplast haplotype frequencies reflects a common history of genetic isolation, followed by recent migration and contact (see below). Evidence for chloroplast introgression The chloroplast haplotypes of P. lambertiana resolve in two different clades in the phylogenetic analysis of Pinus subsect. Strobus, one comprised of five other North American species and the other encompassing 12 Eurasian species and the North American Pinus albicaulis (whitebark pine). Two biological processes could explain these results: incomplete lineage sorting of an ancestral polymorphism, or chloroplast introgression. Incomplete lineage sorting has been determined to be the most probable source of widespread allelic nonmonophyly at nuclear loci in species of Pinus subgenus Strobus (Syring et al. 2007). It has also been considered a potential cause of similar patterns observed in chloroplast studies in other plant species (Tsitrone et al. 2003). However, the stochastic process of incomplete lineage sorting is not expected to show the strong geographical partitioning observed for the two chloroplast haplotypes. On the other hand, if the two subgroups of sugar pine have been separated since the Miocene (15.1 ± 3.8 Ma) and each retained a different haplotype, one might expect to find morphological divergence between (and sequence variation within) the two groups, particularly since this same interval has apparently been accompanied by multiple speciation events in these lineages. Although some sequence divergence was found in the S haplotype clade (in the geographically isolated Baja California population), none was found in the N haplotype clade. The amount of sequence divergence between the S haplotype of P. lambertiana and the other North American white pines is consistent with genetic isolation since the Miocene. In contrast, the high sequence similarity between the N haplotype of sugar pine and the Asian clade is suggestive of a much more recent shared plastid ancestry. To account for this unexpected genetic similarity, we suggest that the N haplotype of P. lambertiana may have its origin in a chloroplast introgression event involving P. albicaulis. Introgression-mediated chloroplast transfer has been named ‘chloroplast capture’ (Rieseberg & Soltis 1991; Tsitrone et al. 2003) and has been offered as an explanation for similar patterns of cytonuclear incongruence observed in many plant genera (reviewed in Rieseberg et al. 1996; Wendel & Doyle 1998). Tsitrone et al. (2003) have modelled the process under the assumption of maternal chloroplast inheritance and they determined conditions likely to promote its occurrence. A key aspect of their model is the observation that cytonuclear incompatibility often results in full or partial male sterility and thus can increase maternal fitness through enhanced seed production. Although they do not explicitly model paternal inheritance (the situation in Pinaceae), they suggest that chloroplast introgression should be less common here, since cytonuclear interactions typically reduce male fitness. Theoretically, chloroplast substitution could result in an advantage in male function, but this situation has apparently not been documented (Tsitrone et al. 2003). However, a pattern consistent with chloroplast introgression has been observed in other species of Pinaceae, e.g. Pinus montezumae (Matos & Schaal 2000), Pinus muricata (Hong et al. 2003) and Larix sibirica (Wei & Wang 2003). Petit et al. (2003) offer ‘pollen swamping’ as an alternative explanation for the lack of cytoplasmic (cpDNA and mito­ chondrial DNA) differentiation between Quercus petraea and Quercus robur, two sympatric oaks that are consistently differentiated at nuclear markers. In their scenario, Q. robur seed disperses into new habitats which are subsequently colonized by Q. petraea via pollen flow, resulting in F1 hybrids. Asymmetric introgression and strong selection for the Q. petraea phenotype results in mixed populations that share a single cytoplasm. Petit et al. (2003) invoke the fact that the seeds of Q. robur are better adapted to birddispersal than Q. petraea, and thus are more likely to establish through long-distance dispersal. A similar relationships exists between P. albicaulis (dispersed mainly by the pine seed specialist, Clark’s nutcracker, Tomback 2005) and P. lambertiana (dispersed to a limited extent by wind and primarily by generalist Steller’s jays and yellow pine chipmunks, Thayer & Vander Wall 2005). In both cases, the better disperser is thought to have contributed its chloroplast to the other species. An important caveat is that the cytoplasm is maternally inherited in oaks, and thus carried by the seed and not by the pollen as in pines. If chloroplast introgression is responsible for this pattern, how does one account for the otherwise ‘Eurasian’ haplotype in two species of North American subsect. Strobus? The cpDNA-based phylogeny (Fig. 3) requires two dispersal events between western North America and Asia. The disjunction between P. monticola and the Eurasian clade can be dated to the Late Miocene or Early Pliocene. This timing is consistent with estimates of 2.6 –16.7 Ma from 11 other eastern Asian/western North American plant disjunctions (Zhu et al. 2006; Zhang et al. 2007). It is © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd P H Y L O G E N Y A N D P H Y L O G E O G R A P H Y 3935 noteworthy that well-preserved Pliocene and Pleistocene fossils of P. monticola have been collected in northeastern Siberia and Alaska (reviewed in Bingham et al. 1972). Following diversification of the Eurasian white pines, and origin of the ‘closed cone’ morphology characteristic of stone pines, we propose that the ancestor of P. albicaulis dispersed from Asia to North America via Beringia, presumably during the Pleistocene. Beringia was largely unglaciated during the Pleistocene, and is known to have served as a refugium for trees and shrubs, including Pinus, through the late glacial maximum (Brubaker et al. 2005). Krutovskii et al. (1995) proposed a similar scenario based on allozyme and chloroplast restriction fragment analysis of the ‘subsect. Cembrae’ pines, but placed the migration at an earlier period (Pliocene). Evidence from mitochondrial DNA haplotypes has been used to infer the existence of three Late Pleistocene refugia for P. albicaulis (Richardson et al. 2002): western Wyoming, western Idaho and the southern Cascades of Oregon. The southern Cascades currently support large populations of P. lambertiana, and we propose that this region could also have served as a northern glacial refugium for sugar pine, or possibly farther west in the Klamath/Siskiyou Mountains (a region with several palaeo-endemic conifers, e.g. Picea breweriana and Chamaecyparis lawsoniana). Sugar pine is common here, but whitebark pine has only recently been discovered in a small population on Mount Ashland (nine individuals; Murray 2005). Regardless of their current geographical distributions, sympatry in a glacial refugium could have provided the opportunity for the northern populations of sugar pine to acquire the chloroplast of whitebark pine. Although P. albicaulis generally occurs at higher altitudes than P. lambertiana, the two are partly sympatric in northern California and southern Oregon (Fig. 1B). There is also evidence that P. lambertiana formerly occurred at higher altitudes than its current distribution (May 1974; Millar et al. 2006). Two factors are required for chloroplast introgression to occur; sympatry and reproductive compatibility. While many interspecific crosses have been conducted in subsect. Strobus, there is no record of attempts to cross P. lambertiana and P. albicaulis (Critchfield 1986; R. Sniezko, personal communication). Critchfield & Kinloch (1986) do, however, document interspecific hybridization between P. lambertiana and Asian members of subsect. Strobus, namely P. armandii and P. koraiensis. Seed set in these artificial crosses averaged 2.1% and 0.2% viable seed per cone, respectively. In contrast, P. lambertiana is apparently intersterile with all other North American white pines (Critchfield 1986; Fernando et al. 2005). No naturally occurring hybrids of P. lambertiana have been recorded (Mirov 1967; Critchfield 1986; R. Sniezko, personal communication). Our results explain why P. lambertiana was resolved in conflicting positions in recent chloroplast sequence-based © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd phylogenetic analyses of pines. Gernandt et al. (2005) sampled P. lambertiana in Oregon (a region fixed for the N haplotype), while Eckert & Hall (2006) sampled an individual from southern California (a region fixed for the S haplo­ type). Each study placed P. lambertiana in a position that is consistent with the resolution of the respective haplotypes in our study (Fig. 3). This demonstrates the importance of sampling multiple individuals per species in phylogenetic analyses of closely related species (see also Syring et al. 2007). The results reported here provide the first phylogeo­ graphical hypothesis for an ecologically and economically important conifer, P. lambertiana. By placing the intraspecific results within a broader phylogenetic context, further insights were gained into the evolutionary history of this species. The novel hypotheses of two Pleistocene refugia for P. lambertiana and chloroplast introgression with P. albicaulis can be tested with additional molecular markers, in particular nuclear and mitochondrial loci. The observation that the two chloroplast haplotypes demarcate population groups that differ in their vulnerability to white pine blister rust is also a significant result that merits further attention. Beyond sugar pine, this study demonstrates the value of including an interspecific phylogenetic component in phylogeographical research. Without this broader per­ spective, the antiquity of the haplotype groups would remain unknown, as would the unexpected, and potentially reticulate, history of these species. Acknowledgements We are indebted to John Gleason (USFS, Placerville Nursery and Disease Resistance Program), Jerry Berdeen and Richard Sniezko (USFS, Dorena Genetic Resource Center) and David Johnson (USFS, Institute of Forest Genetics) for supplying sugar pine seeds and Roman Businsky for providing his collections of Asian species. We thank David Gernandt, Bohun Kinloch, Todd Ott, Paul Severns, Richard Sniezko, and Diana Tomback for constructive comments on the manuscript. This research was funded by National Science Foundation grants DEB 0317103 and ATOL 0629508, an NSF Research Experience for Undergraduates supplement, and the Pacific Northwest Research Station, USDA Forest Service. References Aagaard JE, Krutovskii KV, Strauss SH (1998) RAPD markers of mitochondrial origin exhibit lower population diversity and higher differentiation than RAPDs of nuclear origin in Douglas fir. Molecular Ecology, 7, 801–812. Bingham RT, Hoff RJ, Steinhoff RJ (1972) Genetics of Western White Pine, USDA Forest Service Research Paper WO-12. USDA Forest Service, Washington, D.C. Brubaker LB, Anderson PM, Edwards ME, Lozhkin AV (2005) Beringia as a glacial refugium for boreal trees and shrubs: new perspectives from mapped pollen data. Journal of Biogeography, 32, 833–848. 3936 A . L I S T O N E T A L . Chiang YC, Hung KH, Schaal BA et al. (2006) Contrasting phylo­ geographical patterns between mainland and island taxa of the Pinus luchuensis complex. Molecular Ecology, 15, 765 – 779. Conkle MT (1996) Patterns of variation in isozymes of sugar pine. In: Sugar Pine: Status, Values, and Roles in Ecosystems: Proceedings of a Symposium Presented by the California Sugar Pine Management Committee (eds Kinloch BB, Marosy M, Huddleston ME), p. 99. University of California Division of Agriculture and Natural Resources, Davis, California. Critchfield WB (1986) Hybridization and classification of the white pines (Pinus section Strobus). Taxon, 35, 647 – 656. Critchfield WB, Kinloch BB (1986) Sugar pine and its hybrids. Silvae Genetica, 35, 138 – 145. Critchfield WB, Little EL Jr (1966) Geographic Distribution of the Pines of the World, Forest Service Miscellaneous Publication 991. USDA Forest Service, Washington, D.C. Devey ME, Delfino-Mix A, Kinloch BB, Neale DB (1995) Random amplified polymorphic DNA markers tightly linked to a gene for resistance to white pine blister rust in sugar pine. Proceedings of the National Academy of Sciences, USA, 92, 2066 – 2070. Eckert AJ, Hall BD (2006) Phylogeny, historical biogeography, and patterns of diversification for Pinus (Pinaceae): phylogenetic tests of fossil-based hypotheses. Molecular Phylogenetics and Evolution, 40, 166– 182. Fernando DD, Long SM, Sniezko RA (2005) Sexual reproduction and crossing barriers in white pines: the case between Pinus lambertiana (sugar pine) and P. monticola (western white pine). Tree Genetics and Genomes, 1, 143 – 150. Gadek PA, Alpers DL, Heslewood MM, Quinn CJ (2000) Relationships within Cupressaceae sensu lato: a combined morphological and molecular approach. American Journal of Botany, 87, 1044–1057. Gernandt DS, Gaeda López G, Ortiz García S, Liston A (2005) Phylogeny and classification of Pinus. Taxon, 54, 29 – 42. Hall TA (1999) bioedit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symposium Series, 41, 95 – 98. Hausner G, Olson R, Simon D et al. (2006) Origin and evolution of the chloroplast trnK (matK) intron: a model for evolution of group II intron RNA structures. Molecular Biology and Evolution, 23, 380–391. Hickman JC (1993) Geographic subdivisions of California. In: The Jepson Manual: Higher Plants of California (ed. Hickman JC), pp. 37–48. University of California, Berkeley, California. Hong Y-P, Krupkin AB, Strauss SH (1993) Chloroplast DNA transgresses species boundaries and evolves at variable rates in the California closed-cone pines (P. radiata, P. muricata, and P. attenuata). Molecular Phylogenetics and Evolution, 2, 322–329. Hood GM (2006) PopTools Version 2.7.5. CSIRO, Canberra, Australia. http://www.cse.csiro.au/poptools/. Johansen AD, Latta RG (2003) Mitochondrial haplotype distribution, seed dispersal and patterns of postglacial expansion of ponderosa pine. Molecular Ecology, 12, 293 – 298. Kidd DM, Ritchie MG (2006) Phylogeographic information systems: putting the geography into phylogeography. Journal of Biogeography, 33, 1851 – 1865. Kinloch BB (1992) Distribution and frequency of a gene for resistance to white pine blister rust in natural populations of sugar pine. Canadian Journal of Botany, 70, 1319 – 1323. Kinloch BB (2003) White pine blister rust in North America: past and prognosis. Phytopathology, 93, 1044 – 1047. Kinloch BB, Scheuner WH (2004) Pinus lambertiana Dougl., Sugar Pine. USDA Forest Service, Washington, D.C. http://na.fs.fed.us/ spfo/pubs/silvics_manual/Volume_1/vol1_Table_of_contents.htm. Krutovskii KV, Politov DV, Altukhov YP (1995) Isozyme study of population genetic structure, mating system and phylogenetic relationships of the five stone pine species (subsection Cembrae, section Strobi, subgenus Strobus). In: Population Genetics and Genetic Conservation of Forest Trees (eds Baradat P, Adams WT, Müller-Starck G), pp. 279–304. Academic Publishing, Amsterdam. Latta RG, Mitton JB (1999) Historical separation and present gene flow through a zone of secondary contact in ponderosa pine. Evolution, 53, 769–776. Ledig FT (1998) Genetic variation in Pinus. In: Ecology and Biogeog­ raphy of Pinus (ed. Richardson DM), pp. 251–280. Cambridge University Press, Cambridge, UK. Liston A (1992) Variation in the chloroplast genes rpoC1 and rpoC2 of the genus Astragalus (Fabaceae): evidence from restriction site mapping of a PCR-amplified fragment. American Journal of Botany, 79, 953–961. Liston A, Robinson WA, Piñero D, Alvarez-Buylla ER (1999) Phylogenetics of Pinus (Pinaceae) based on nuclear ribosomal DNA internal transcribed spacer sequences. Molecular Phylo­ genetics and Evolution, 11, 95–109. Martinson SR (1997) Association among geographic, allozyme, and growth variables for sugar pine (Pinus lambertiana Dougl.) in southwest Oregon and throughout the species range. PhD Thesis, North Carolina State University. Matos JA, Schaal BA (2000) Chloroplast evolution in the Pinus montezumae complex: a coalescent approach to hybridization. Evolution, 54, 1218–1233. May RH (1974) An observation of some sugar pine relicts. Madroño, 22, 276. Mielke JL (1943) White Pine Blister Rust in Western North America, Bulletin no. 52, Yale University School of Forestry, New Haven, Connecticut. Millar CI, King JC, Westfall RD, Alden HA, Delany DL (2006) Late Holocene forest dynamics, volcanism, and climate change at Whitewing Mountain and San Joaquin Ridge, Mono County, Sierra Nevada, CA, USA. Quaternary Research, 66, 273–287. Mirov NT (1967) The Genus Pinus. The Ronald Press Co., New York. Mohr JA, Whitlock C, Skinner CN (2000) Postglacial vegetation and fire history, eastern Klamath mountains, California, USA. Holocene, 10, 587–601. Murray M (2005) Our threatened timberlines: the plight of whitebark pine ecosystems. Kalmiopsis, 12, 25–29. Muse SV, Weir BS (1992) Testing for equality of evolutionary rates. Genetics, 132, 269–276. Navascues M, Vaxevanidou Z, Gonzalez-Martinez SC et al. (2006) Chloroplast microsatellites reveal colonization and metapopu­ lation dynamics in the Canary Island pine. Molecular Ecology, 15, 2691–2698. Neale DB, Sederoff RR (1989) Paternal inheritance of chloroplast DNA and maternal inheritance of mitochondrial DNA in loblolly pine. Theoretical and Applied Genetics, 77, 212–216. Petit RJ, Bodénès C, Ducousso A, Roussel G, Kremer A (2003) Hybridization as a mechanism of invasion in oaks. New Phytologist, 161, 151–164. Petit RJ, Duminil J, Fineschi S et al. (2005) Comparative organization of chloroplast, mitochondrial and nuclear diversity in plant populations. Molecular Ecology, 14, 689–701. Posada D, Crandall KA (1998) modeltest: testing the model of DNA substitution. Bioinformatics, 14, 817–818. © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd P H Y L O G E N Y A N D P H Y L O G E O G R A P H Y 3937 Richardson BA, Brunsfeld SJ, Klopfenstein NB (2002) DNA from bird-dispersed seed and wind-disseminated pollen provides insights into postglacial colonization and population genetic structure of whitebark pine (Pinus albicaulis). Molecular Ecology, 11, 215–227. Richardson DM, Rundel PW (1998) Ecology and biogeography of Pinus: an introduction. In: Ecology and Biogeography of Pinus (ed. Richardson DM), pp. 3 – 46. Cambridge University Press, Cambridge, UK. Rieseberg LH, Soltis DE (1991) Phylogenetic consequences of cytoplasmic gene flow in plants. Evolutionary Trends in Plants, 5, 65–84. Rieseberg LH, Whitton J, Linder CR (1996) Molecular marker incongruence in plants hybrid zones and phylogenetic trees. Acta Botanica Neerlandica, 45, 243 – 262. Rozas J, Sánchez-De I, Barrio JC, Messeguer X, Rozas R (2003) dnasp, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics, 19, 2496 – 2497. Scharpf RF (1993) Diseases of Pacific Coast Conifers, Handbook 521. USDA Forest Service, Washington D.C. Shaw J, Lickey EB, Beck JT et al. (2005) The tortoise and the hare II: relative utility of 21 noncoding chloroplast DNA sequences for phylogenetic analysis. American Journal of Botany, 92, 142–166. Soltis DE, Gitzendanner MA, Strenge DD, Soltis PS (1997) Chloro­ plast DNA intraspecific phylogeography of plants from the Pacific Northwest of North America. Plant Systematics and Evolution, 206, 353– 373. Swofford DL (2002) PAUP*, Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. Sinauer Associates, Sunderland, Massachusetts. Syring J, Farrell K, Businsky R, Cronn R, Liston A (2007) Wide­ spread genealogical nonmonophyly in species of Pinus subgenus Strobus. Systematic Biology, 56, 163 – 181. Syring J, Willyard A, Cronn R, Liston A (2005) Evolutionary relationships among Pinus (Pinaceae) subsections inferred from multiple low-copy nuclear loci. American Journal of Botany, 92, 2086–2100. Thayer TC, Vander Wall SB (2005) Interactions between Steller’s jays and yellow pine chipmunks over scatter-hoarded sugar pine seeds. Journal of Animal Ecology, 74, 364 – 375. Thompson RS, Anderson KH (2000) Biomes of western North America at 18 000, 6000 and 0 14C yr bp reconstructed from pollen and packrat midden data. Journal of Biogeography, 27, 555–584. © 2007 The Authors Journal compilation © 2007 Blackwell Publishing Ltd Tomback DF (2005) The impact of seed dispersal by Clark’s nutcracker on whitebark pine: multi-scale perspective on a high mountain mutualism. In: Mountain Ecosystems: Studies in Treeline Ecology (eds Broll G, Keplin B), pp. 181–201. Springer, Berlin. Tsitrone A, Kirkpatrick M, Levin DA (2003) A model for chloro­ plast capture. Evolution, 57, 1776–1782. Wang XR, Tsumura Y, Yoshimaru H, Nagasaka K, Szmidt AE (1999) Phylogenetic relationships of Eurasian pines (Pinus, Pinaceae) based on chloroplast rbcL, matK, rpl20-rps18 spacer, and trnV intron sequences. American Journal of Botany, 86, 1742 – 1753. Wefald M (2001) TRS2LL, http://www.geocities.com/jeremiahobrien/ trs2ll.html. Wei XX, Wang XQ (2003) Phylogenetic split of Larix: evidence from paternally inherited cpDNA trnT-trnF region. Plant Systematics and Evolution, 239, 67–77. Wendel JF, Doyle JJ (1998) Phylogenetic incongruence: window into genome history and molecular evolution. In: Molecular Systematics of Plants: DNA Sequencing (eds Soltis DE, Soltis PS, Doyle JJ), pp. 265–296. Kluwer, Boston, Massachusetts. Willyard A, Syring J, Gernandt DS, Liston A, Cronn R (2007) Fossil calibration of molecular divergence infers a moderate mutation rate and recent radiations for Pinus. Molecular Biology and Evolution, 24, 90–101. Zhang M-L, Uhink CH, Kadereit JW (2007) Phylogeny and bio­ geography of Epimedium/Vancouveria (Berberidaceae): western North American–East Asian disjunctions, the origin of Euro­ pean mountain plant taxa, and East Asian species diversity. Systematic Botany, 32, 81–92. Zhu Y-P, Wen J, Zhang Z-Y, Chen Z-D (2006) Evolutionary relationships and diversification of Stachyuraceae based on sequences of four chloroplast markers and the nuclear ribosomal ITS region. Taxon, 55, 931–940. Aaron Liston and Rich Cronn collaboratively study the system­ atics, population genetics, and evolution of pines. John Syring and Ann Willyard are former PhD students of Cronn and Liston. Dr Syring is now an Assistant Professor of Plant Systematics at Montana State University-Billings, and Dr Willyard is a postdoc at the University of South Dakota. Mariah Parker-deFeniks is a Sociology major at Oregon State University, pursuing a career in Criminal Justice and Social Research.