Aseptic Processing of Sterile Products: Innovations, Regulatory Guidance and
advertisement
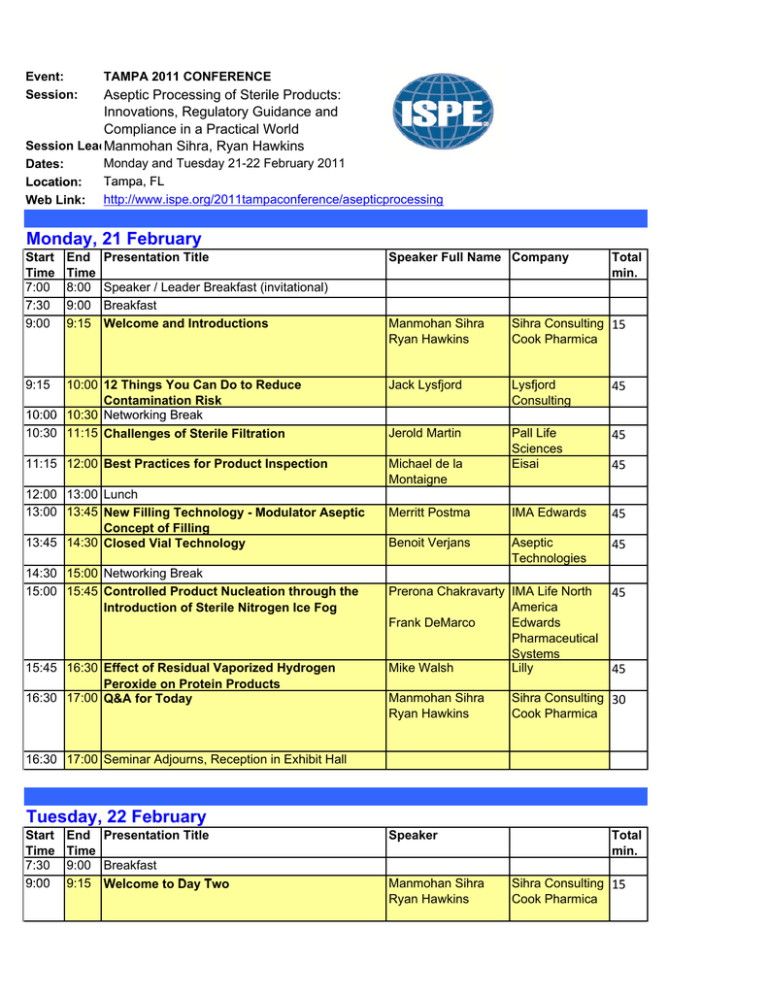
Event: Session: TAMPA 2011 CONFERENCE Dates: Location: Web Link: Monday and Tuesday 21-22 February 2011 Tampa, FL http://www.ispe.org/2011tampaconference/asepticprocessing Aseptic Processing of Sterile Products: Innovations, Regulatory Guidance and Compliance in a Practical World Session LeadManmohan Sihra, Ryan Hawkins Monday, 21 February Start Time 7:00 7 30 7:30 9:00 End Time 8:00 9:00 9 00 9:15 Presentation Title Speaker / Leader Breakfast (invitational) Breakfast B kf Welcome and Introductions Speaker Full Name Company Total min. Manmohan Sihra Ryan Hawkins Sihra Consulting 15 Cook Pharmica 10:00 12 Things You Can Do to Reduce Contamination Risk 10:00 10:30 Networking Break 10:30 11:15 Challenges of Sterile Filtration Jack Lysfjord Lysfjord Consulting 45 Jerold Martin 45 11:15 12:00 Best Practices for Product Inspection Michael de la Montaigne Pall Life Sciences Eisai Merritt Postma IMA Edwards 45 Benoit Verjans Aseptic Technologies 45 9:15 12:00 13:00 Lunch 13:00 13:45 New Filling Technology - Modulator Aseptic Concept of Filling 13:45 14:30 Closed Vial Technology 14:30 15:00 Networking Break 15:00 15:45 Controlled Product Nucleation through the Introduction of Sterile Nitrogen Ice Fog 15:45 16:30 Effect of Residual Vaporized Hydrogen Peroxide on Protein Products 16:30 17:00 Q&A for Today Prerona Chakravarty IMA Life North America Frank DeMarco Edwards Pharmaceutical S Systems Mike Walsh Lilly Manmohan Sihra Ryan Hawkins 45 45 45 Sihra Consulting 30 Cook Pharmica 16:30 17:00 Seminar Adjourns, Reception in Exhibit Hall Tuesday, 22 February Start Time 7:30 9:00 End Time 9:00 9:15 Presentation Title Breakfast W l t D T Welcome to Day Two Speaker Manmohan Sihra Ryan Hawkins Total min. Sihra Consulting 15 Cook Pharmica 9:15 10:00 Isolator Filling Line Case Study 10:00 10:30 Networking Break 10:30 11:15 Gowning Challenges for Aseptic Operations Ryan Hawkins Cook Pharmica 45 Joerg Zimmerman Vetter 45 11:15 12:00 Single-use Disposable Systems (SUDs) for Aseptic Fill/Finish: Concepts and Application Rob Roy Jason Collins IPS IPS 45 12:00 12 00 13:00 13 00 Lunch L h 13:00 13:45 An Overview of the updated ISPE Sterile Guide Mark von Stwolinski CRB Consulting This is an overview of the second edition of ISPE’s Engineers Baseline Pharmaceutical Engineering Guide for New and Renovated Facilities Volume 3 ‘Sterile Manufacturing Facilities’ which was originally published in 1999. ISPE Guides aim to describe current good practices that can help p p a company p y develop p an approach pp that is effective, cost-efficient and in compliance with existing regulations and related guidance, while allowing a flexible and innovative approach to facility design, construction, commissioning, and qualification. The key items presented will be: • Identifying the individuals involved with these updates • The process for getting to this second edition • What has change in the Guide and why •H How this thi updated d t d Guide G id can help h l better b tt assist i t the th industry 13:45 14:30 Update on PDA Aseptic Process Simulations Technical Report (TR22) Revisions 14:30 15:00 Networking Break 15:00 15:45 Environmental Monitoring in Aseptic Operation 15:45 16:30 Effect of Advances in Aseptic on Regulation 16 30 17:00 16:30 17 00 Q&A ffor T d Today 17:00 Seminar Adjourns Tony Pavell APP Pharma 45 Michael Miller 45 Jim Akers Microbiology Consultants Akers Kennedy M Manmohan h Sihra Sih Ryan Hawkins Sih Sihra C Consulting lti 30 Cook Pharmica 45 0