CRYSTAL CHEMISTRY Wh i l h
advertisement

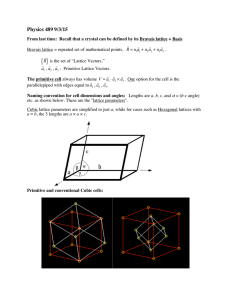
CRYSTAL CHEMISTRY
Wh iis crystall chemistry?
What
h i
?
Reading:
West 7, 8
• description and classification of crystals
• composition-structure
iti
t t
relationships
l ti
hi
• the conditions in which particular type of crystal structure is observed
• structure-property
structure property relations
Common ways to describe crystals:
• unit cell approach (specify size, shape and atomic positions)
• close-packing approach (good for metals
metals, alloys
alloys, ionic structures
structures, covalent
networks, molecular and supermolecular solids)
space-filling
g po
polyhedron
y ed o app
approach
oac
• space
63
CLOSE-PACKED CRYSTAL STRUCTURES
Consider the close-packing of incompressible (hard) spheres:
In 2D, regular close-packing requires an hexagonal array (HCP)
Most efficient way
to pack spheres of single size
• 6 nearest neighbors
Coordination number (CN): 6
In 3D, regular close-packing involves stacking 2D HCP arrays
CCP
Regular
(crystalline)
packing
ki
Irregular
packing
64
HEXAGONAL CLOSE-PACKED STRUCTURE
An HCP crystal is a close-packed structure with the stacking sequence ...ABABAB...
ABABAB
To construct:
1st layer: 2D HCP array (layer A)
2ndd layer:
l
HCP layer
l
with
i h each
h sphere
h placed
l d iin alternate
l
interstices
i
i
in
i 1stt layer
l
(B)
3rd layer: HCP layer positioned directly above 1st layer (repeat of layer A)
…ABABABAB…
A
B
A
B
A
A
HCP is two interpenetrating simple hexagonal
lattices displaced by a1/3 + a2/3 + a3/2
65
HCP STRUCTURE
• not a Bravais
avais lattice
Orientation alternates
with each layer
• each sphere touches 12 equidistant nearest neighbors (CN = 12)
Six in plane, six out-of-plane
• structure has maximum p
packingg ffraction possible
p
for
f single-sized
g
spheres
p
((0.74))
66
HCP STRUCTURE
• ideal ratio c/a of
8 / 3 1.633
• unit cell is a simple hexagonal lattice with a
two-point basis
a
(0 0 0)
(0,0,0)
a
(2/3,1/3,1/2)
• {0002} planes are close packed
• ranks in importance with FCC and BCC
Bravais lattices
Plan view
67
HCP STRUCTURE
• about 30 elements crystallize in the HCP form
68
CUBIC CLOSE-PACKED STRUCTURE
A CCP crystal is a close-packed structure with the stacking sequence ...ABCABC...
ABCABC
To construct:
1st layer: 2D HCP array (layer A)
2ndd layer:
l
HCP layer
l
with
i h each
h sphere
h placed
l d iin alternate
l
interstices
i
i
in
i 1stt layer
l
(B)
3rd layer: HCP layer placed in the other set of interstitial depressions (squares, C)
4th layer: repeats the 1st layer (A)
…ABCABCABC…
ABCABCABC
stacking
g
of HCP layers
along
body diagonals
A
B
C
It tturns outt th
thatt th
the CCP structure
t t
is
i just
j t
the FCC Bravais lattice!
69
CCP STRUCTURE
• CN = 12
12, packing fraction 0.74
0 74
• {111} planes are close packed
• 4 atoms in unit cell
Plan view
70
CLOSE-PACKED STRUCTURES
• most common are
a e HCP
C and CC
CCP
• an infinite # of alternative stacking sequences exist
Example: silicon carbide has over 250 polytypes
e.g., 6H-SiC
stacking sequence …ABCACB…
71
STACKING FAULTS
Stacking faults are one or two layer interruptions in the stacking sequence that
destroy lattice periodicity
e.g., an <110> projection of an FCC lattice:
[111]
[110]
[110]
[001]
A
B
C
A
B
C
A
B
C
A
B
C
B
C
A
B
C
perfect FCC
ABCABCABC
missing
l
plane
of atoms
faulted FCC
ABCBCABC
The stacking fault is an example of a planar defect
• stacking fault energy γ ~100 mJ m-22
• results also in perpendicular linear defects called dislocations
72
EXAMPLE
InAs nanowires - <110> projection
Caroff, P. et al. Nature Nanotechnology 4, 50 - 55 (2009)
73
• CCP and HCP have veryy similar lattice energies
g
• no clear cut trends
74
Nature
353, 147 - 149 (12 Sep 1991)
Rare Gases:
G
Ne, He, Ar,
A Kr, Xe (CCP)
(CC )
75
gold nanocrystals
X. M. Lin
76
ANOTHER VIEW OF CLOSE PACKING
77
. It was
reviewed by a panel of 12 referees; the panel reported in 2003
2003, after 4 years of
work, that it was “99% certain” of the correctness of the proof, but couldn’t
verify the correctness of all of the computer calculations. Hales and Ferguson
(his student) received the Fulkerson Prize for outstanding papers in the area of
discrete mathematics in 2009.
http://en.wikipedia.org/wiki/Kepler_conjecture
78
PACKING FRACTIONS
The fraction of the total crystal volume that is occupied by spheres
CCP (and HCP)
radius
4 a 23
4
)
(
Vatoms
fraction
3 3 4 2 0.7405
Vcell
a
6
a 2
a
a 2
4
74%
BCC
SC
a 3
radius
4
4 a 33
2 (
)
fraction 3 3 4 0.6802
0 6802
a
68%
4 a3
( )
fraction 3 32 0.5236
a
52%
79
80
DENSITY CALCULATION
n: number
b off atoms/unit
/ i cell
ll
matoms
nA
Vcell
VC N A
A: atomic mass
VC: volume of the unit cell
NA: Avogadro’s number
(6.023x1023 atoms/mole)
Calculate the density of copper.
RCu =0.128nm, Crystal structure: FCC, ACu= 63.5 g/mole
n = 4 atoms/cell,,
VC a 3 ((2 2 R )3 16 2 R 3
(4)(63.5)
3
g
cm
8.89
/
[16 2(1.28 108 )3 (6.023 1023 )]
8.96 g/cm3 in the literature
81
INTERSTICIAL SITES IN CP STRUCTURES
A large number of ionic structures can be regarded as built of CP layers of anions
with the cations placed in interstitial sites
for everyy anion,, there is 1 Octahedral site and 2 Tetrahedral sites
82
Octahedral holes
coordinates:
di t
½00
0½0
00½
½½½
= O site
cavities have <100>
orientation
i t ti
83
Tetrahedral holes in CCP
T+ sites:
¾¼¼
¼¾¼
¼¼¾
¾¾¾
T- sites:
¼¼¼
¾¾¼
¼¾¾
¾¼¾
cavities have <111> orientation
84
Holes in HCP
O sites:
2/3,1/3,1/4
2/3,1/3,3/4
, ,
T+ sites:
1/3
1/3,2/3,1/8
2/3 1/8
0,0,5/8
(1/3,2/3,1/2)
(0,0,0)
a2
a1
T- sites:
0,0,3/8
, ,
1/3,2/3,7/8
85
LOCATION OF OCTAHEDRAL HOLES
86
LOCATION OF TETRAHEDRAL HOLES
(3/8 of a unit cell directly
above/below each anion)
87
SIZE OF OCTAHEDRAL CAVITY
Onlyy cations smaller than the diameter of the cavity
y can fit
without forcing the anion lattice to expand
from cell edge
a 2rM 2rX
from face diagonal
M = cation
ti
X = anion
a 2 2rX
.
The cation radius must be
< 41% of the anion radius
88
SIZE OF TETRAHEDRAL CAVITY
The tetrahedral holes are twice as
numerous but six times smaller in volume
from face diagonal
g
a 2 / 2 2rX
from body diagonal
a 3 / 2 2rM 2rX
The cation radius must be
< 23% of the anion radius
89
EUTACTIC STRUCTURES
Structures in which the arrangement of ions is the same as in a close packed array
but the ions are not necessarily touching
Within certain loose limits (given by the radius ratio rules), cations
too large to fit in the interstices can be accommodated by an
expansion of the anion array
• anions don’t like to touch anyway
• modern techniques show that, in many cases, anions (cations) are
not as large
g ((small)) as ppreviouslyy thought
g
• we still describe eutactic structures as CCP or HCP lattices
with ions in some fraction of the interstitial sites
90
CRYSTALS THAT CAN BE DESCRIBED IN
TERMS OF INTERSTITIAL FILLING OF A
CLOSE-PACKED STRUCTURE
91
SOME EUTACTIC CRYSTAL STRUCTURES
Variables:
1)
2)
anion layer stacking sequence: CCP or HCP array?
occupancy of interstitial sites
92
93
NaCl (ROCK SALT, HALITE) STRUCTURE
(CCP 100% O
(CCP,
Oct.
t H
Holes
l Fill
Filled)
d)
Space Group = Fm3m
Lattice = FCC
Basis = Cl (0,0,0), Na (½,½,½)
Coordination = 6, 6
Cation Coord. → Octahedron
Anion Coord.
Coord → Octahedron
Connectivity → Edge sharing octahedra
with faces parallel to {111}
4 NaCl in unit cell
94
POLYHEDRAL REPRESENTATION
• shows the topology and indicates interstitial sites
• tetrahedra and octahedra are the most common shapes
Rock Salt:
• Array of edge sharing NaCl6 octahedra
• Each
h octahedron
h d
shares
h
allll 12 edges
d
• Tetrahedral interstices
Galena (PbS)
95
ROCK SALT - OCCURANCE
• Very common (inc. 'ionics', 'covalents' & 'intermetallics' )
• Most alkali
lk l halides
h l
(CsCl,
(
l CsBr, CsI excepted))
• Most oxides / chalcogenides of alkaline earths
• Many
M n nit
nitrides,
id s carbides,
bid s h
hydrides
d id s ((e.g. Z
ZrN,
N TiC
TiC, N
NaH)
H)
96
COMPLEX ION VARIANT OF ROCK SALT
• space group = Pa3
• sulfur dimers oriented along <111>
97
ZINC BLENDE (ZnS, SPHALERITE)
(CCP T+
(CCP,
T Holes
H l Fill
Filled)
d)
Space Group = F43m
Lattice = FCC
B i = S (0,0,0),
Basis
(0 0 0) Zn
Z (¼,¼,¼)
(¼ ¼ ¼)
Coordination = 4, 4
Cation Coord. → Tetrahedron
Anion Coord. → Tetrahedron
Connectivity → Corner sharing Tetra.
4 ZnS in unit cell
98
ZINC BLENDE
GaAs
• bonding is less ionic than in rock salt
• common for Be, Zn, Cd, Hg chalcogenides (i.e., ZnS, ZnSe, ZnTe)
• common for III-V compounds (B, Al, Ga, In with N, P, As, Sb)
99
DIAMOND STRUCTURE
S
Same
as sphalerite,
h l it but
b t with
ith identical
id ti l atoms
t
in
i all
ll positions
iti
Space Group = F43m
Lattice = FCC
B i = C (0,0,0),
Basis
(0 0 0) C (¼,¼,¼)
(¼ ¼ ¼)
Coordination = 4
g Tetra.
Connectivityy → Corner sharing
8 C atoms per unit cell
100
FLUORITE (CaF2) & ANTIFLUORITE (Na2O)
Fluorite : CCP of Ca2+, 100% Tetra. Holes Filled with FAnti-fluorite : cation and anion positions are reversed
Ca2+
Space Group = Fm3m
Lattice = FCC
Basis = Ca2+ (0,0,0),
(0 0 0) F- (¼,¼,¼)
(¼ ¼ ¼) & (¾
(¾,¾,¾)
¾ ¾)
Coordination = 8, 4 (fluorite)
Cation Coord. → Cubic
Anion Coord. → Tetrahedral
Connectivity → Edge sharing FCa4 tetrahedra or edge sharing CaF8 cubes
4 CaF2 in unit cell
101
ALTERNATIVE REPRESENTATIONS
Ca2+
Displacing the unit cell by ¼ of a body diagonal emphasizes the cubic cation coordination:
F-
102
FLUORITE / ANTIFLUORITE
Ca2+
• origin of the term “fluorescence” (1852)
• fluorite
fl it common for
f fluorides
fl id off large,
l
divalent cations and oxides of large
tetravalent cations (M2+F2 and M4+O2)
• antifluorite common for
oxides/chalcogenides of alkali earths (M2O)
CaF2
103
FLUORESCENT MINERALS
= fluorite
104
http://en.wikipedia.org/wiki/Fluorescence
COMPARING NaCl, ZnS, Na2O
NaCl
ZnS
Na2O
105
106
Li3Bi EXAMPLE
107
NiAs STRUCTURE
(HCP, 100% Oct. Holes Filled)
2
Space Group = P63/mmc
Lattice = Primitive hexagonal
Basis = As (0,0,0) & (2/3,1/3,1/2)
Ni (1/3,2/3,1/4) & (1/3,2/3,3/4)
Coordination = 6, 6
Cation Coord. → Octahedron
Anion Coord.
Coord → Trigonal prism
Connectivity → Edge/face sharing Oct.
or edge-sharing trigonal prisms
2 NiAs in unit cell
108
Alternative unit cell with Ni at the origin:
109
NiAs
•Transition
Transition metals with
chalcogens, As, Sb, Bi
e.g. Ti(S,Se,Te);
Cr(S,Se,Te,Sb);
( , , , )
Ni(S,Se,Te,As,Sb,Sn)
110
WURTZITE (ZnS) STRUCTURE
(HCP T
(HCP,
T+ Holes
H l Fill
Filled)
d)
Space Group = P63mc
Lattice = Primitive hexagonal
B i = S (0,0,0)
Basis
(0 0 0) & (2/3,1/3,1/2)
(2/3 1/3 1/2)
Zn (0,0,5/8) & (2/3,1/3,1/8)
Coordination = 4, 4
Cation Coord. → Tetrahedron
Anion Coord. → Tetrahedron
Connectivity → Corner sharing Tetra.
2 ZnS in unit cell
111
ZnO
Projections perpendicular to close-packed planes
112
113
Very different next-nearest neighbor coordinations & beyond
114
HCP VERSION OF CaF2?
N structures
No
t
t
are known
k
with
ith all
ll T
Tetra.
t
sites
it (T
(T+ and
d T-)
T ) fill
filled
d iin HCP
- i.e. there is no HCP analogue of the Fluorite /Anti-Fluorite structure
Why?
The T+ and T- interstitial
sites above and below a layer
of close-packed spheres in
HCP are too close to each
other (distance = 0.25c) to
tolerate the coulombic
repulsion generated by filling
with like-charged ions.
Face-linking is unfavorable
115
RUTILE STRUCTURE (TiO2)
((distorted HCP,, 50% Oct. Holes Filled))
Ti
O
(0,0,0)
Space Group = P42/mnm
Lattice = Primitive tetragonal
Basis = Ti (0,0,0) & (½,½,½)
O (0
(0.3,0.3,0),
3 0 3 0) (0
(0.7,0.7,0),
7 0 7 0) (0
(0.8,0.2,0.5),
8 0 2 0 5) (0
(0.2,0.8,0.5)
2 0 8 0 5)
Coordination = 6, 3
Cation Coord. → Octahedral
Anion Coord. → Trigonal planar
Connectivity → chains of edge-sharing Oct.
along c axis, linked by vertices
2 TiO2 per unit cell
116
ANATASE STRUCTURE (TiO2)
(di t t d CCP,
(distorted
CCP 50% Oct.
O t Holes
H l Filled)
Fill d)
Ti
O
a = 3.776 Å
b=3
3.776
776 Å
c = 9.486 Å
Volume anatase TiO2: 136.25 Å3
rutile TiO2: 62.07 Å3
Space Group = I41/amd
Lattice = body-centered tetragonal
Coordination = 6, 3
Cation Coord. → Octahedral
Anion Coord. → Trigonal planar
Connectivity → chains of edge-sharing
edge sharing Oct.
Oct
along c axis, linked by vertices
4 TiO2 per unit cell
117
RUTILE AND ANATASE
chains of edge sharing oct.,
linked at corners
greater density of edge sharing
→ a bit less stable
118
CdI2 STRUCTURE
(HCP with
(HCP,
ith Cd in
i Oct.
O t Holes
H l off alternate
lt
t layers)
l
)
A layered crystal
Cd
I
Space Group = P3m1
Lattice = Primitive trigonal
B i = Cd (0,0,0)
Basis
(0 0 0)
I (2/3,1/3,1/4) & (1/3,2/3,3/4)
Coordination = 6, 3
Cation Coord. → Octahedron
Anion Coord. → Trigonal pyramid
Connectivity → sheets of edge-sharing Oct.
1 CdI2 per unit cell
119
Alternative
unit cell
with Cd at
the origin:
CdI6 units
NiAs6 units
120
CdI2 - OCCURANCE
• Iodides of moderately polarizing cations; bromides and
chlorides of
f strongly
g y polarizing
p
g cations;;
e.g. PbI2, FeBr2, VCl2
• Hydroxides of many divalent cations
e.g. (Mg,Ni)(OH)2
• Di-chalcogenides of many quadrivalent cations
e.g. TiS2, ZrSe
Z S 2, CoTe
C T 2
121
CdCl2 STRUCTURE
The CCP analogue of CdI2
(CCP, with Cd in Oct. Holes of alternate layers along [111])
CdCl6 octahedra
Space Group = R32/m
Chlorides of moderately polarizing cations
e.g. MgCl2, MnCl2
Di-sulfides of quadrivalent cations
e.g. TaS2, NbS2 (CdI2 form as well)
122
Formula
Type and fraction
of sites occupied
CCP
HCP
AB
All octahedral
NaCl
Rock Salt
NiAs
Nickel Arsenide
Half
tetrahedral
(T+ or T-)
ZnS
Zinc Blende
ZnS
Wurtzite
A2B
All tetrahedral
Na2O Anti-Fluorite
N
A ti Fl it
CaF2 Fluorite
not known
A3B
All octahedral
& ttetrahedral
t h d l
Li3Bi
not known
AB2
Half octahedral
(Alternate layers
full/empty)
CdCl2 (Cadmium Chloride)
CdI2 (Cadmium Iodide)
Half octahedral
(Ordered
framework
arrangement)
TiO2 (Anatase)
CaCl2
TiO2 (Rutile)
Third octahedral
Alternate layers
2/ full/empty
3
YCl3
BiI3
AB3
123
PEROVSKITE STRUCTURE ABO3 (CaTiO3)
(CCP of Ca & O , 25% Oct. Holes Filled by Ti)
A-Cell
B C ll
B-Cell
Space Group = Pm3m
Lattice = Primitive cubic
B i = Ti (0
Basis
(0,0,0),
0 0) Ca
C (½,½,½),
(½ ½ ½)
O (½,0,0), (0,1/2 ,0) & (0,0,½)
Coordination = Ca-12 ; Ti-6; O-6
Ca Coord. → Cuboctahedron
Ti Coord. → Octahedron
O Coord. → distorted octahedron (4 Ca, 2 Ti)
1 CaTiO3 per unit cell
An extremely important class of ABX3
compounds:
Magnetoresistance
Ferroelectricity
Multiferroics
Superconductivity
d
Catalysis (fuel cells)
Spin transport
124
PEROVSKITE CONNECTIVITY
B-Cell
3D network of corner-sharing
octahedra
Network of face-sharing
cuboctahedra
125
Perovskites: the most widely studied oxide structure
• Wide range of chemistries possible
- thousands of examples known
• Cubic, tetragonal, and orthorhombic symmetries are common
Unique properties of perovskites
- high Tc cuprate superconductors
- Colossal Magneto-Resistance
Magneto Resistance (La,SrMnO
(La SrMnO3)
- fast ion conduction (Li+, O2-), batteries, fuel cells
- mixed electronic/ionic conduction, fuel cells
- oxidation/reduction catalysts
- ferroelectric / piezoelectric ceramics (BaTiO3, Pb(ZrTi)O3)
- important mineral structure in lower mantle (MgSiO3, pyroxene)
- frequency
q
y filters for wireless communications : Ba(Zn
( 1/3Ta2/3))O3
126
X
A
Perovskite Structure: ABX3
Tolerance factor (t):
rA rX
t
2(rB rX )
B
A-Cell
t
>1
0.9 - 1.0
Effect
A cation too large to fit in
interstices
ideal
0.71 - 0.9 A cation too small
< 0.71
A cation same size as B cation
Likely structure
Hexagonal
perovskite
Cubic perovskite
Orthorhombic
perovskite
Possible close packed
127
lattice
PEROVSKITES
Most perovskites contain distorted octahedra and are not cubic
cubic. These distortions give
perovskites a rich physics.
symmetry at 25
25°C
C
BaTiO3: Ba2+
Ti4+
O2-
r = 1.56 Å
r = 0.68 Å
r = 1.26 Å
t = 1.03 - tetragonal
KNbO3:
K+
1.65 Å
Nb5+ 0.78 Å
t = 1.01 - orthorhombic
LiNbO3:
Li+
1.06 Å
Nb5+ 0.78 Å
t = 0.81 – trigonal
LiNbO3 : ferroelectricity, Pockels effect, piezoelectricity, photoelasticity,
nonlinear optical polarizability
128
129
Reading: West 15
C m-2
V m-11
130
DI- , PARA- , AND FERROELECTRICS
response
p
of atom to applied
pp
E field
dipole moment: p = qd = αE
polarization: P = Σp/V
P = ε0χeE
p
dielectric polarization
P : polarization (C/m2)
ε0: vacuum permittivity – 8.85 x 10-12 C2 N-1 m-2
χe: electric
l
susceptibility
bl
((unitless)
l
)
E : electric field (V/m, or N/C)
paraelectric polarization
• linear: P = ε0χeE
• nonlinear
• no P without E
• no P without E
ferroelectric polarization
• residual (zero-field)
(zero field) polarization
• reversible direction of residual P
• very large susceptibilities
131
WHY IS BaTiO3 FERROELECTRIC
ferroelectric phase transition
~0.1 Å displacement
> 120°C cubic, not FE
< 120°C tetragonal, FE
transition occurs at the
Curie temperature, Tc
132
dielectric constant
εr = χ e + 1
133
FERROELECTRIC HYSTERESIS LOOPS
remnant polarization, PR
saturation polarization, Ps
dipoles aligned “up”
coercive field
field, EC
dipoles aligned “down”
134
ORDERED ELECTRIC DIPOLE PHASES
ferroelectric (BaTiO3)
• parallel ordering below Tc
antiferroelectric (PbZrO3)
• antiparallel ordering below Tc
• E field can induce
ferroelectric state
ferrielectric (Bi4Ti3O12)
• net spontaneous polarization in only certain direction(s)
135
CURIE TEMPERATURE
Thermal energy destroys the ordered electric dipole state
state. The temperature above
which this order-disorder phase transition occurs is the Curie temperature, Tc.
Above Tc, the material is often paraelectric.
ordered
F / AF
randomized
orientation
P
Note:
These curves
omit the
“spikes” in P
at Tc
136
PHASE DIAGRAMS
137
K2NiF4 STRUCTURE (La2CuO4)
Many “complex”
complex structures are composed of simple,
simple familiar building blocks
blocks.
The high-Tc copper oxide superconductors are an example.
Doped La2CuO4 was the first (1986) High-Tc Superconducting Oxide (Tc ~ 40 K)
B d
Bednorz
& Müller
Müll were awarded
d daN
Nobel
b lP
Prize
i
La2CuO4 may be viewed as if constructed from an ABAB... arrangement of
Perovskite cells - known as an AB Perovskite!
B
A
B
2 La2CuO4 per unit cell
138
ALTERNATE VIEWS OF La2CuO4
We may
y view the structure as based on:
1. Sheets of elongated CuO6 octahedra, sharing only vertices
2. Layered networks of CuO46-, connected only by La3+ ions
139
COMMON STRUCTURAL FORM
Cations form
FCC with O22interstitials
• Common structural motif of vertex-linked CuO4 squares
• This motif occurs in all the high-TC superconducting copper oxides
• The structures differ in the structure of the 'filling' in the 'sandwich‘
of copper oxide layers - known as Intergrowth Structures
140
YBa2Cu3O7: THE 1,2,3 SUPERCONDUCTOR
• the first material to superconduct at LN2 temperature, Tc > 77 K
• YBa2Cu3O7 can be viewed as an Oxygen-Deficient Perovskite
141
POLYHEDRAL REPRESENTATION OF YBCO
Two types
T
t p s of
f Cu
C sites:
sit s:
1) Layers of CuO5 square pyramids
2) Chains of vertex-linked CuO4 squares
CuO2
BaO
CuO
BaO
CuO2
Y
CuO2
142
SPINEL STRUCTURE AB2O4 (MgAl2O4)
(CCP, Mg in 1/8th of Tetra. Holes and Al in 50% of Oct. Holes)
a = 8.08 Å
Space Group = Fd3m
Lattice = FCC
Coordination = Mg-4; Al-6; O-4
Mg Coord. → Tetrahedron
Al Coord. → Octahedron
Connectivity → chains of Edge-sharing AlO6
octahedra, linked by MgO 4 tetra.
8 MgAl2O4 per unit cell (56 atoms)
• extremely flexible structure, adopted
by over 100 compounds
• normal spinel: 8 A in Tetra., 16 B in Oct.
• inverse spinel: 8 A in Oct., 8 B in Oct. and
8 B in Tetra.
• intermediate cations distributions also
143
occur.
144
SPINELS - OCCURANCE
Aluminium spinels:
Spinel – MgAl2O4, after which this
class of minerals is named
Gahnite - ZnAl2O4
H
Hercynite
i - FeAl
F Al2O4
Iron spinels:
Magnetite - Fe3O4
Franklinite - (Fe,Mn,Zn)(Fe,Mn)2O4
Ulvöspinel - TiFe2O4
Jacobsite - MnFe2O4
Trevorite - NiFe2O4
Ch
Chromium
i
spinels:
s i ls:
Chromite - FeCr2O4
Magnesiochromite - MgCr2O4
Others with the spinel
p
structure:
Ulvöspinel - Fe2TiO4
Ringwoodite - Mg2SiO4, an abundant
olivine polymorph within the Earth's
mantle from about 520 to 660 km
depth, and a rare mineral in
meteorites
145
CRYSTAL FIELD STABILIZATION ENERGY
In transition metal compounds,
compounds d electron effects such as crystal field
stabilization energy (CFSE) can be important in determining structure.
crystal field splitting diagrams
e.g. MF2 compounds (high spin rutile)
Δoct =
Δtetra =
CFSEoct = (0.4 × #t2g – 0.6 × #eg) Δoct
Δtetra = (4/9)Δoct
No CFSE
146
CATION SITE PREFERENCES IN SPINELS
The larger CFSE of metal ions in octahedral sites is sometimes an important
f
factor
in d
determining spinell structures ((normall vs inverse).
)
Normal - [A]tet[B2]octO4
Inverse - [B]tet[A,B]octO4
γ = fraction of A in oct. sites
γ = 0 is normal, γ = 1 is inverse
In the absence of CFSE effects: 2,3 spinels tend to be normal (MgAl2O4)
4,2 spinels tend to be inverse (TiMg2O4)
In 2,3 spinels,
l CFSE
Ef
favors the
h f
following:
ll
1) Chromium spinels (Cr3+) are normal
2) Magnetite (Fe3O4) is inverse b/c
Fe3+ has zero CFSE, while Fe2+ prefers oct.
3) Mn3O4 is normal b/c Mn2+ has no CFSE
147
CORUNDUM STRUCTURE (α-Al2O3)
(HCP 2/3 off Oct.
(HCP,
O t Holes
H l filled)
fill d)
Space Group = R3c
Lattice = Primitive trigonal
Coordination = 6, 4
Cation Coord.
Coord → Octahedron
Anion Coord. → distorted tetrahedron
Connectivity → edge, face-sharing Oct.
6 Al2O3 per unit cell
• Ruby (Cr), sapphire (Fe, Ti, Cr), Fe2O3
148