An Experimental Study of Carbon Dioxide Desorption from a
advertisement
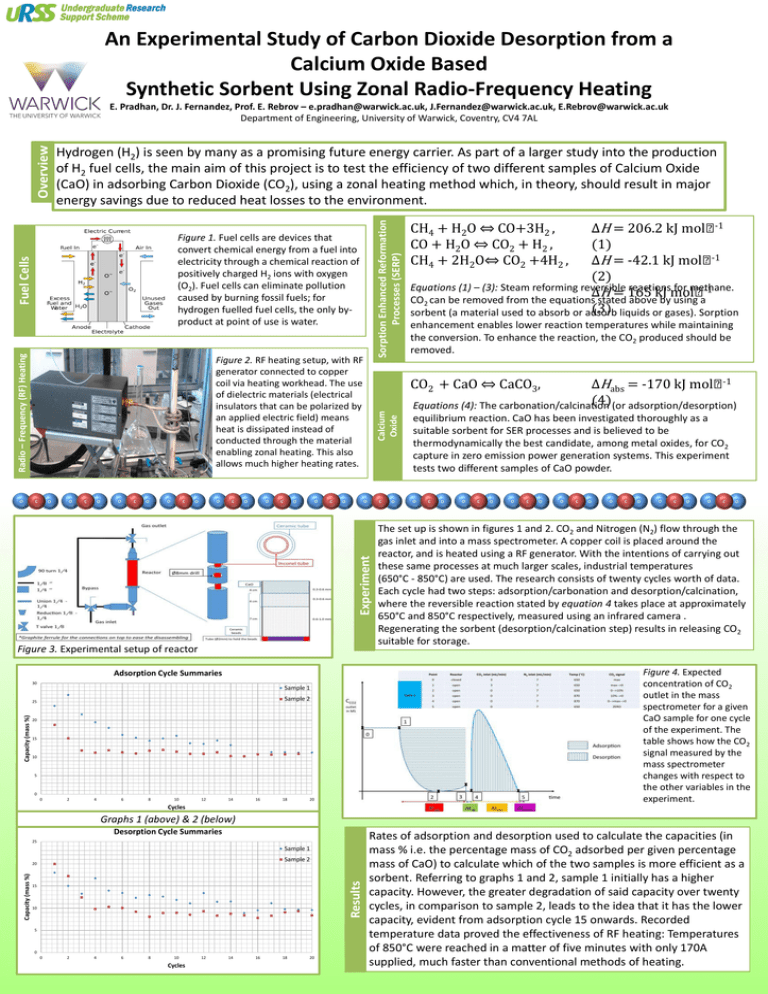
An Experimental Study of Carbon Dioxide Desorption from a Calcium Oxide Based Synthetic Sorbent Using Zonal Radio-Frequency Heating Hydrogen (H2) is seen by many as a promising future energy carrier. As part of a larger study into the production of H2 fuel cells, the main aim of this project is to test the efficiency of two different samples of Calcium Oxide (CaO) in adsorbing Carbon Dioxide (CO2), using a zonal heating method which, in theory, should result in major energy savings due to reduced heat losses to the environment. Sorption Enhanced Reformation Processes (SERP) Overview E. Pradhan, Dr. J. Fernandez, Prof. E. Rebrov – e.pradhan@warwick.ac.uk, J.Fernandez@warwick.ac.uk, E.Rebrov@warwick.ac.uk Department of Engineering, University of Warwick, Coventry, CV4 7AL Radio – Frequency (RF) Heating Fuel Cells Figure 1. Fuel cells are devices that convert chemical energy from a fuel into electricity through a chemical reaction of positively charged H2 ions with oxygen (O2). Fuel cells can eliminate pollution caused by burning fossil fuels; for hydrogen fuelled fuel cells, the only byproduct at point of use is water. Figure 2. RF heating setup, with RF generator connected to copper coil via heating workhead. The use of dielectric materials (electrical insulators that can be polarized by an applied electric field) means heat is dissipated instead of conducted through the material enabling zonal heating. This also allows much higher heating rates. Sample 1 Sample 2 Capacity (mass %) 25 20 15 10 5 0 0 2 4 6 8 10 12 14 16 18 ΔHabs = -170 kJ mol-1 (4) (or adsorption/desorption) Equations (4): The carbonation/calcination Calcium Oxide equilibrium reaction. CaO has been investigated thoroughly as a suitable sorbent for SER processes and is believed to be thermodynamically the best candidate, among metal oxides, for CO2 capture in zero emission power generation systems. This experiment tests two different samples of CaO powder. The set up is shown in figures 1 and 2. CO2 and Nitrogen (N2) flow through the gas inlet and into a mass spectrometer. A copper coil is placed around the reactor, and is heated using a RF generator. With the intentions of carrying out these same processes at much larger scales, industrial temperatures (650°C - 850°C) are used. The research consists of twenty cycles worth of data. Each cycle had two steps: adsorption/carbonation and desorption/calcination, where the reversible reaction stated by equation 4 takes place at approximately 650°C and 850°C respectively, measured using an infrared camera . Regenerating the sorbent (desorption/calcination step) results in releasing CO2 suitable for storage. Figure 4. Expected concentration of CO2 outlet in the mass spectrometer for a given CaO sample for one cycle of the experiment. The table shows how the CO2 signal measured by the mass spectrometer changes with respect to the other variables in the experiment. Adsorption Cycle Summaries 30 enhancement enables lower reaction temperatures while maintaining the conversion. To enhance the reaction, the CO2 produced should be removed. CO2 + CaO ⇔ CaCO3, Experiment Figure 3. Experimental setup of reactor ΔH = 206.2 kJ mol-1 (1) ΔH = -42.1 kJ mol-1 (2) Equations (1) – (3): Steam reforming reversible reactions for methane. ΔH = 165 kJ mol-1 CO2 can be removed from the equations stated above by using a (3) liquids or gases). Sorption sorbent (a material used to absorb or adsorb CH4 + H2O ⇔ CO+3H2 , CO + H2O ⇔ CO2 + H2 , CH4 + 2H2O⇔ CO2 +4H2 , 20 Cycles Graphs 1 (above) & 2 (below) Desorption Cycle Summaries 25 Sample 1 Sample 2 Results Capacity (mass %) 20 15 10 5 0 0 2 4 6 8 10 Cycles 12 14 16 18 20 Rates of adsorption and desorption used to calculate the capacities (in mass % i.e. the percentage mass of CO2 adsorbed per given percentage mass of CaO) to calculate which of the two samples is more efficient as a sorbent. Referring to graphs 1 and 2, sample 1 initially has a higher capacity. However, the greater degradation of said capacity over twenty cycles, in comparison to sample 2, leads to the idea that it has the lower capacity, evident from adsorption cycle 15 onwards. Recorded temperature data proved the effectiveness of RF heating: Temperatures of 850°C were reached in a matter of five minutes with only 170A supplied, much faster than conventional methods of heating.