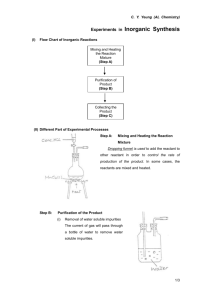
2-2.2.2 Evaporation of the Liquid
advertisement
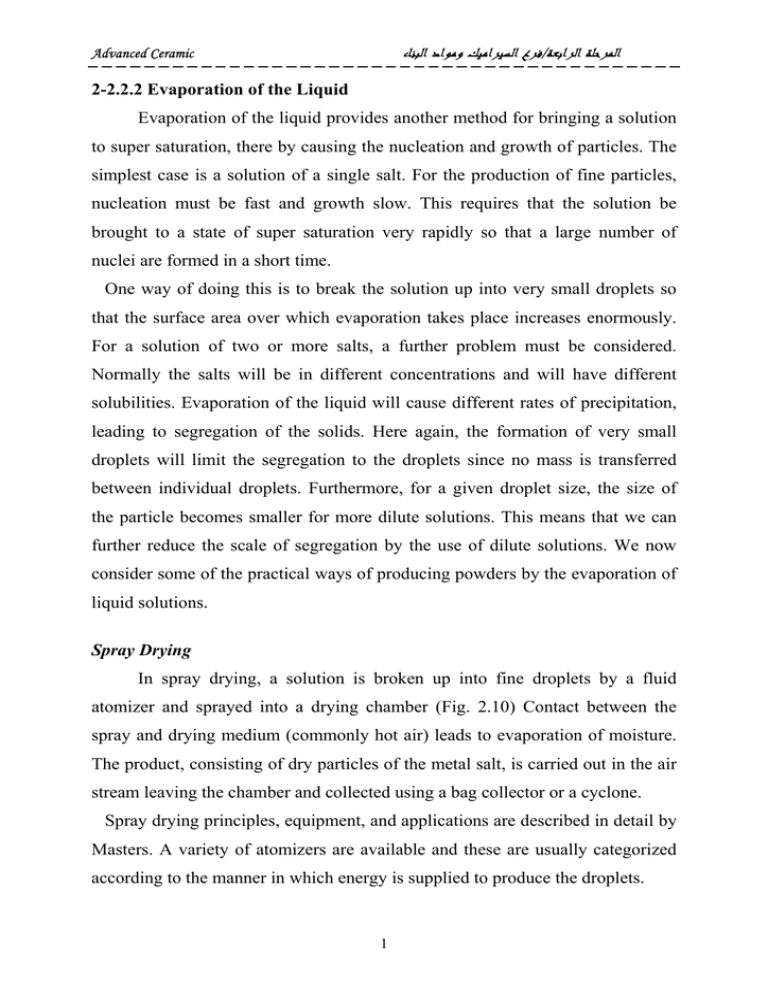
ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺮﺍﺑﻌﺔ Advanced Ceramic 2-2.2.2 Evaporation of the Liquid Evaporation of the liquid provides another method for bringing a solution to super saturation, there by causing the nucleation and growth of particles. The simplest case is a solution of a single salt. For the production of fine particles, nucleation must be fast and growth slow. This requires that the solution be brought to a state of super saturation very rapidly so that a large number of nuclei are formed in a short time. One way of doing this is to break the solution up into very small droplets so that the surface area over which evaporation takes place increases enormously. For a solution of two or more salts, a further problem must be considered. Normally the salts will be in different concentrations and will have different solubilities. Evaporation of the liquid will cause different rates of precipitation, leading to segregation of the solids. Here again, the formation of very small droplets will limit the segregation to the droplets since no mass is transferred between individual droplets. Furthermore, for a given droplet size, the size of the particle becomes smaller for more dilute solutions. This means that we can further reduce the scale of segregation by the use of dilute solutions. We now consider some of the practical ways of producing powders by the evaporation of liquid solutions. Spray Drying In spray drying, a solution is broken up into fine droplets by a fluid atomizer and sprayed into a drying chamber (Fig. 2.10) Contact between the spray and drying medium (commonly hot air) leads to evaporation of moisture. The product, consisting of dry particles of the metal salt, is carried out in the air stream leaving the chamber and collected using a bag collector or a cyclone. Spray drying principles, equipment, and applications are described in detail by Masters. A variety of atomizers are available and these are usually categorized according to the manner in which energy is supplied to produce the droplets. 1 ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺮﺍﺑﻌﺔ Advanced Ceramic In rotary atomization (often referred to as centrifugal atomization), the liquid is centrifugally accelerated to high velocity by a spinning disk located at the top of the drying chamber before being discharged into the chamber. In pressure atomization, pressure nozzles atomize the solution by accelerating it through a large pressure difference and injecting it into the chamber. Pneumatic atomization occurs when the solution is impacted by a stream of high-speed gas from a nozzle. Ultrasonic atomization involves passing the solution over a piezoelectric device that is vibrating rapidly. Droplet sizes ranging from less than 10 µm to over 100 µm can be produced by these atomizers. The solutions in spray drying are commonly aqueous solutions of metal salts. Sulfates and chlorides are often used because of their high solubility. In the drying chamber, the temperature and flow pattern of the hot air as well as the design of the chamber determine the rate of moisture removal from the droplet and the maximum temperature (typically less than ~300°C) that the particles will experience. The key solution parameters are the size of the droplet and the concentration and composition of the metal salt. These parameters control the primary particle size and the size and morphology of the agglomerate. The morphology of the agglomerate is not very critical in spray drying of solutions because the particle characteristics are largely determined by subsequent calcination and milling steps. Under suitable conditions, spherical agglomerates with a primary particle size of ~0.1 µm or less can be obtained. Because the temperature in the drying chamber is commonly insufficient to cause decomposition or solid-state reaction, the spray-dried salt must be subjected to additional processing steps such as calcination and milling to achieve suitable characteristics for processing. Spray drying of solutions has been found to be useful for the preparation of ferrite powders. For Ni-Zn ferrite, the solution of sulfates was broken up into droplets (10–20 µm) by a rotary atomizer. The powder obtained by spray drying was in the form of hollow spheres having the same size as the original droplets. 2 ﻓﺮﻉ ﺍﻟﺴﻴﺮﺍﻣﻴﻚ ﻭﻣﻮﺍﺩ ﺍﻟﺒﻨﺎﺀ/ﺍﻟﻤﺮﺣﻠﺔ ﺍﻟﺮﺍﺑﻌﺔ Advanced Ceramic Calcination at 800–1000°C produced a fully reacted powder consisting of agglomerates with a primary particle size of ~0.2 µm. The ground powder (Particle size < 1 µm) was compacted and sintered to almost theoretical density. FIGURE 2.10 Schematic of a spray dryer for the production of powders. 3