line, and the steam is allowed to enter the cylinder... 8-84
advertisement

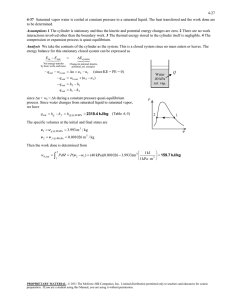
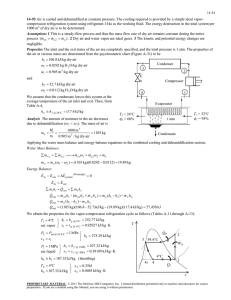
8-75 8-84 An insulated cylinder initially contains saturated liquid-vapor mixture of water. The cylinder is connected to a supply line, and the steam is allowed to enter the cylinder until all the liquid is vaporized. The amount of steam that entered the cylinder and the exergy destroyed are to be determined. Assumptions 1 This is an unsteady process since the conditions within the device are changing during the process, but it can be analyzed as a uniform-flow process since the state of fluid at the inlet remains constant. 2 The expansion process is quasi-equilibrium. 3 Kinetic and potential energies are negligible. 4 The device is insulated and thus heat transfer is negligible. Properties The properties of steam are (Tables A-4 through A-6) h1 h f x1 h fg 561.43 0.8667 2163.5 2436.5 kJ/kg x1 13 / 15 0.8667 s1 s f x1 s fg 1.6716 0.8667 5.3200 6.2824 kJ/kg K P1 300 kPa P2 300 kPa h2 h g @ 300 kPa 2724.9 kJ/kg sat.vapor s 2 s g @ 300 kPa 6.9917 kJ/kg K Pi 2 MPa hi 3248.4 kJ/kg Ti 400C s i 7.1292 kJ/kg K Analysis (a) We take the cylinder as the system, which is a control volume. Noting that the microscopic energies of flowing and nonflowing fluids are represented by enthalpy h and internal energy u, respectively, the mass and energy balances for this unsteady-flow system can be expressed as H2O 300 kPa P = const. 2 MPa 400C m in m out m system m i m 2 m1 Mass balance: Energy balance: E E inout Net energy transfer by heat, work, and mass E system Change in internal, kinetic, potential, etc. energies mi hi W b,out m 2 u 2 m1u1 (since Q ke pe 0) Combining the two relations gives 0 W b,out m 2 m1 hi m 2 u 2 m1u1 0 m 2 m1 hi m 2 h2 m1 h1 or, since the boundary work and U combine into H for constant pressure expansion and compression processes. Solving for m2 and substituting, m2 Thus, hi h1 (3248.4 2436.5)kJ/kg m1 (15 kg) = 23.27 kg hi h2 (3248.4 2724.9)kJ/kg mi m 2 m1 23.27 15 8.27 kg (b) The exergy destroyed during a process can be determined from an exergy balance or directly from its definition X destroyed T0 S gen where the entropy generation Sgen is determined from an entropy balance on the insulated cylinder, S S out in Net entropy transfer by heat and mass S gen S system Entropy generation Change in entropy mi s i S gen S system = m 2 s 2 m1 s1 S gen m 2 s 2 m1 s1 mi s i Substituting, the exergy destruction is determined to be X destroyed T0 S gen T0 [m 2 s 2 m1 s1 mi s i ] (298 K)(23.27 6.9917 15 6.2824 8.27 7.1292) 2832 kJ PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.