P determined; and an expression for thermal efficiency as functions of... 9-24
advertisement

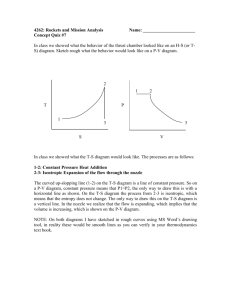
9-17 9-24 An air-standard cycle executed in a piston-cylinder system is composed of three specified processes. The cycle is to be sketcehed on the P-v and T-s diagrams; the heat and work interactions and the thermal efficiency of the cycle are to be determined; and an expression for thermal efficiency as functions of compression ratio and specific heat ratio is to be obtained. Assumptions 1 The air-standard assumptions are applicable. 2 Kinetic and potential energy changes are negligible. 3 Air is an ideal gas with constant specific heats. Properties The properties of air are given as R = 0.3 kJ/kg·K and cv = 0.3 kJ/kg·K. Analysis (a) The P-v and T-s diagrams of the cycle are shown in the figures. (b) Noting that c p cv R 0.7 0.3 1.0 kJ/kg K k cp cv P 1.0 1.429 0.7 3 2 Process 1-2: Isentropic compression v T2 T1 1 v2 k 1 T1 r k 1 1 (293 K )(5) 0.429 584.4 K v w1 2,in cv (T2 T1 ) (0.7 kJ/kg K )(584.4 293) K 204.0 kJ/kg q12 0 T From ideal gas relation, T3 v 3 v 1 r T3 (584.4)(5) 2922 T2 v 2 v 2 Process 2-3: Constant pressure heat addition 3 3 2 1 s w2 3,out Pdv P2 (v 3 v 2 ) R(T3 T2 ) 2 (0.3 kJ/kg K )(2922 584.4) K 701.3 kJ/kg q 23,in w2 3,out u 23 h23 c p (T3 T2 ) (1 kJ/kg K )(2922 584.4) K 2338 kJ/kg Process 3-1: Constant volume heat rejection q 31,out u13 c v (T3 T1 ) (0.7 kJ/kg K)(2922 - 293) K 1840.3 kJ/kg w31 0 (c) Net work is wnet w2 3,out w1 2,in 701.3 204.0 497.3 kJ/kg K The thermal efficiency is then th wnet 497.3 kJ 0.213 21.3% 2338 kJ q in PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission. 9-18 (d) The expression for the cycle thermal efficiency is obtained as follows: th wnet w23,out w1 2,in q in q in R (T3 T2 ) c v (T2 T1 ) c p (T3 T2 ) c v (T1 r k 1 T1 ) R c p c p (rT1 r k 1 T1 r k 1 ) T1 c v T1 r k 1 1 T r k 1 R 1 cp c p T1 r k 1 (r 1) T1 1 R 1 c p k (r 1) T1 r k 1 1 1 R 1 c p k (r 1) r k 1 1 1 1 1 1 k 1 ( 1 ) k k r r since c R c p cv 1 1 v 1 cp cp cp k PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.