The power output of the engine and the source temperature... Then the power output of this heat engine can be...
advertisement
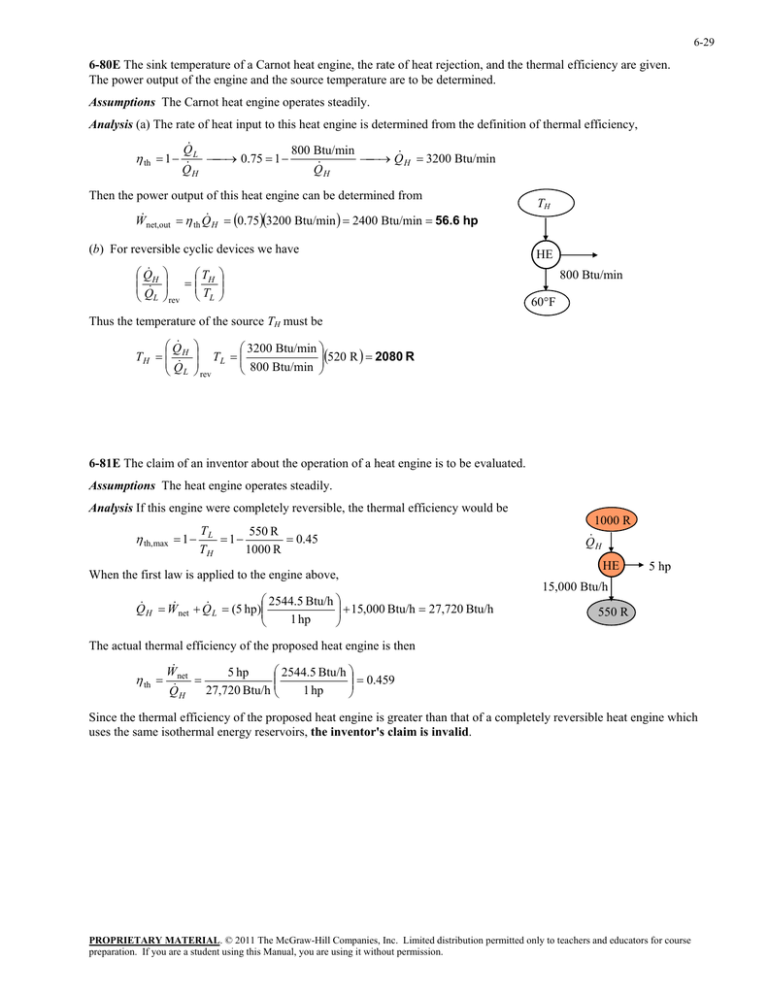
6-29 6-80E The sink temperature of a Carnot heat engine, the rate of heat rejection, and the thermal efficiency are given. The power output of the engine and the source temperature are to be determined. Assumptions The Carnot heat engine operates steadily. Analysis (a) The rate of heat input to this heat engine is determined from the definition of thermal efficiency, th 1 Q L 800 Btu/min 0.75 1 Q H 3200 Btu/min Q Q H H Then the power output of this heat engine can be determined from W net,out th Q H 0.753200 Btu/min 2400 Btu/min 56.6 hp (b) For reversible cyclic devices we have Q H Q L T H rev TL TH HE 800 Btu/min 60°F Thus the temperature of the source TH must be Q TH H Q L 3200 Btu/min TL 800 Btu/min 520 R 2080 R rev 6-81E The claim of an inventor about the operation of a heat engine is to be evaluated. Assumptions The heat engine operates steadily. Analysis If this engine were completely reversible, the thermal efficiency would be th,max T 550 R 1 L 1 0.45 TH 1000 R When the first law is applied to the engine above, Q H W net 2544.5 Btu/h 15,000 Btu/h 27,720 Btu/h Q L (5 hp) 1 hp 1000 R Q H HE 5 hp 15,000 Btu/h 550 R The actual thermal efficiency of the proposed heat engine is then th W net 2544.5 Btu/h 5 hp 0.459 27,720 Btu/h 1 hp QH Since the thermal efficiency of the proposed heat engine is greater than that of a completely reversible heat engine which uses the same isothermal energy reservoirs, the inventor's claim is invalid. PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.