REAXYS MEDICINAL CHEMISTRY THE REAXYS CHEMISTRY DISCOVERY ENGINE
advertisement
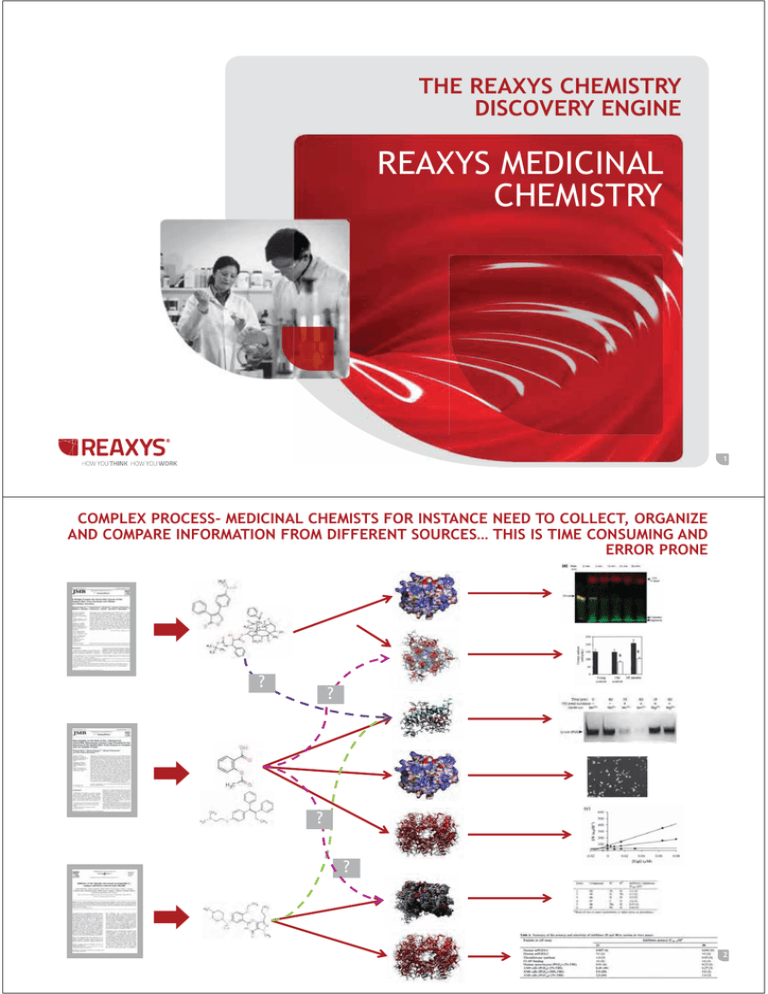
THE REAXYS CHEMISTRY DISCOVERY ENGINE REAXYS MEDICINAL CHEMISTRY 1 COMPLEX PROCESS- MEDICINAL CHEMISTS FOR INSTANCE NEED TO COLLECT, ORGANIZE AND COMPARE INFORMATION FROM DIFFERENT SOURCES… THIS IS TIME CONSUMING AND ERROR PRONE ? ? ? ? 2 AND TO MAKE A DECISION ON WHICH EXPERIMENT TO DO, YOU WANT TO ORGANIZE YOUR CONTENT, NORMALIZE AND COMPARE, TO UNDERSTAND WHICH COMPOUND INTERACTS WITH WHICH TARGET AND TRIGGER WHICH BIOLOGICAL EFFECT—THAT'S A LOT OF MANUAL WORK! 9 9 9 8 9 8 8 8 9 8 9 8 9 8 9 8 8 8 8 8 9 9 9 9 9 3 WHAT IS REAXYS MEDICINAL CHEMISTRY? GOAL Enable better decisions to be made throughout drug discovery process Better select the most promising compounds to progress into the clinic Abandon the wrong compounds earlier HOW Access millions of chemical compounds Search biological experimental results Thousands of druggable proteins Intuitive user interface Powerful underlying classification and organisation BECOME More effective More efficient More quickly ACHIEVE Find and optimise a promising chemical series Explore the pharmacological effect of selected compounds Investigate the targets with which the compound series interacts Use for the assessment of new drugs, compound repurposing, lead identification and lead optimisation 4 ACCESS ESSENTIAL INFORMATION ALIGNED TO MEDICINAL CHEMISTS, PHARMACOLOGISTS AND COMPUTATIONAL CHEMISTS WORKFLOW Compound Chemical structure ,Name, code, synonym of compound Druggable target Explore Target affinity patterns of chemical compounds In vitro and Cell Based assays In vitro assays (binding, second messenger etc..) and Cell based assays for example : Aggregation, Angiogenesis, Apoptosis, Cell differentiation, Cellular Cycle, Chondrogenesis Animal models disease ovariectomized rat in osteoporosis, treatment of glaucoma, Xenografted animals with tumors to test and develop antineplastic drugs Pharmacokinetic and ADME Properties Metabolic stability, Intrinsic clearance, Half life of elimination, Bioavailability, In vivo Clearance Toxicity Cytotoxicity, cardiotoxicity, chronic toxicity 5 RMC 2 CONTENT Patents Origin and starting date >80 000 Patents US : 1971-present EP : 1979-present WO : 1978-present (English only) Patents are coming from the A61K class mainly but not only. « The most comprehensive medicinal chemistry solution on the market » Articles and Journals >1000 Journals Covered >300 000 Articles From 1980 to Present Drugable Targets >7000 Drugable Targets 4.5M Cpds 20M bioactivities Extended Content Integrated GOSTAR data 6 ESSENTIAL INFORMATION: 100+ EXPERIMENTAL FIELDS Reaxys Medicinal Chemistry excerpts all the relevant Quantitative data 7 RMC KEY FEATURES 8 REAXYS MEDICINAL CHEMISTRY- INTUITIVE UI REAXYS-LIKE MEDICINAL CHEMISTRY OPTIMIZED QUERY/HOME PAGE 9 RESULTS VIEW - HEATMAP Filters to narrow down Manage X and Y axis Bioactivity data based on pX values 10 PX CONCEPT ? Parameter Filter IC50,Ki,% Inhibition, %,EC50,pKi,ED50,pIC50,AUC,Emax(%), Concentration,Cmax,nH,pA2,% Stimulation,Tmax,Fold increase,t1/2 el,Rate,Number,KD,pEC50,pKb,IA (%),Time,Km,ID50,Delta, Vmax,Cl,Clint,Ue (%),pD2,% Max,Kb,Bmax,Cavg,Pressure,Amount,t1/2, Cl/F,Cmin,MED,fu,F(%),Dose,ClR,AUC i/AUC,LD50,Frequency PARAMETERS RELATED TO CONCENTRATION pIC50, pEC50, pED50, pID50, pLC50, pLD50, pKi, pKd, pKb, pD2, pD’2, pA2 , IC50, EC50, ED50, ID50, LC50, LD50, Ki, Kd, Kb, Ka, Ke , % Inhibition Parameter Grinder pIC50, pEC50, pED50, pID50, pLC50, pLD50, pKi, pKd, pKb, pD2, pD’2, pA2 IC50, EC50, ED50, ID50, LC50, LD50, Ki, Kd, Kb, Ka, Ke , % Inhibition pX 11 REAXYS MEDICINAL CHEMISTRY & REAXYS- INTEROPERABILITY 12 REAXYS & REAXYS MEDICINAL CHEMISTRY INTEGRATION WITH REAXYS * illustrative prototype - actual implementation may look different 13 DRUG DISCOVERY: THE DREAM TEAM! Substance Search Integrated Bioactivity Data In Vitro Data In Vivo Data Competitive Intelligence Literature Search Reaction Conditions Synthetic Tractability Structure Search Chemistry Biological Profiling Background information Novelty Search Synthetic Route Planning Literature Search 14 USE CASES 15 ACCESS TO KNOWLEDGE ALONG DRUG DISCOVERY CHAIN Project Kick off Indications for target Compound active on target Most active compounds (IC50, Ki, EC50 < 50nM) Most active competitors Target selectivity PK of Compounds classes Adverse effects : CYPblockade Adverse effect : hERGactivity Compound Library Focused Library hERG Model CYP Model HTS/Virtual Screening Hit to Lead Calcium T Type Channel High affinity towards the target Hit Generation : customer story Show selectivity versus targets Phenotypic in silico Screening Reduce binding to HAS Improve cell permeability Not be metabolized rapidly Not interfere with the P450 enzymes Not interfere with the Pgp Show selectivity versus targets (Advanced) Multiple inhibitors : Renin angiotensin pathway Lead Optimization Exploration of structural features of a lead series of Compounds Safety pharmacology (off Targets) Pharmacokinetics ADMET Computational chemistry and molecular modeling 16 Project Kick off – AKT1 Inhibitors 17 WHICH SUBSTANCES ARE THE MOST ACTIVES ON MY TARGET (HUMAN) OF INTEREST ? Scenario (New Project) NEW PROJECT FOCUSED ON FINDING NEW AKT1 INHIBITORS WITH LESS AFFINITY ON AKT2 (MINIMIZING ADVERSE EFFECT) AKT IS ASSOCIATED WITH TUMOR CELL SURVIVAL, PROLIFERATION, AND INVASIVENESS. THE ACTIVATION OF AKT IS ALSO ONE OF THE MOST FREQUENT ALTERATIONS OBSERVED IN HUMAN CANCER AND TUMOR CELLS. Akt1 has been implicated as a major factor in many types of cancer Akt2 is an important signaling molecule in the Insulin signaling pathway The role of Akt3 is less clear, though it appears to be predominantly expressed in the brain Search for active chemotypes on AKT1? 18 SEARCH FOR ACTIVE CHEMOTYPES ON AKT1? Simple Target name search returns all results All compounds tested on Akt1 Targets on which AKT1 inhibitors were also tested on 19 SEARCH FOR ACTIVE CHEMOTYPES ON AKT1? HUMAN SPECIES Retrieve compounds tested on human AKT1 Compounds tested on Human AKT1 are also tested on AKT2, AKT3 ERK2 and PDK1 20 SEARCH FOR ACTIVE CHEMOTYPES ON AKT1? VERY ACTIVE COMPOUNDS Answers 16 compounds and 18 experimental data Filter on very active compound pX>=9 21 Hit to lead – caspase-based apoptosis 22 WHAT IS KNOWN ABOUT MY SUBSTANCE OF INTEREST? Scenario An Apoptosis inducer ‘chemotype’ from a cell- and caspase-based apoptosis high-throughput screening was found (Compound 1). A structure activity relationship expansion lead to compound 2 (Schema1) Compound 1 Compound 2 HTS hit SAR expansion COC1=CC=C(C=C1)N(C)C1=NC(Cl)=NC2=C1C=CC=C2 What is known about this chemotype/template (Compound 2) in Reaxys Medicinal Chemistry? 23 WHICH ARE THE KNOWN ACTIVITIES OF MY CHEMOTYPE ON OTHER TARGET CLASSES ? Scenario (Hit to lead) An Apoptosis inducer ‘chemotype’ from a cell- and caspase-based apoptosis high-throughput screening was found (Compound 1). A structure activity relationship expansion lead to compound 2 (Schema1) Compound 1 Compound 2 HTS hit SAR expansion COC1=CC=C(C=C1)N(C)C1=NC(Cl)=NC2=C1C=CC=C2 Which are the known activities of my Chemotype on other target classes in Reaxys Medicinal Chemistry? 24 THANK YOU ANY QUESTIONS? DR CHARLES MARTINEZ SOLUTION SALES MANAGER TEL: +447769301845 EMAIL: C.MARTINEZ@ELSEVIER.COM TECHNICAL SUPPORTNLINFO@ELSEVIER.COM JOIN THE BETA 25 EXTRA SLIDES 26 RESULTS VIEW - HEATMAP Filters to narrow down Manage X and Y axis Bioactivity data based on pX values 27 PX CONCEPT ? Parameter Filter IC50,Ki,% Inhibition, %,EC50,pKi,ED50,pIC50,AUC,Emax(%), Concentration,Cmax,nH,pA2,% Stimulation,Tmax,Fold increase,t1/2 el,Rate,Number,KD,pEC50,pKb,IA (%),Time,Km,ID50,Delta, Vmax,Cl,Clint,Ue (%),pD2,% Max,Kb,Bmax,Cavg,Pressure,Amount,t1/2, Cl/F,Cmin,MED,fu,F(%),Dose,ClR,AUC i/AUC,LD50,Frequency PARAMETERS RELATED TO CONCENTRATION pIC50, pEC50, pED50, pID50, pLC50, pLD50, pKi, pKd, pKb, pD2, pD’2, pA2 , IC50, EC50, ED50, ID50, LC50, LD50, Ki, Kd, Kb, Ka, Ke , % Inhibition Parameter Grinder pIC50, pEC50, pED50, pID50, pLC50, pLD50, pKi, pKd, pKb, pD2, pD’2, pA2 IC50, EC50, ED50, ID50, LC50, LD50, Ki, Kd, Kb, Ka, Ke , % Inhibition pX 28 PX CONCEPT COMPETITIVE ADVANTAGE pX it’s a unique way of quantifying affinity of compounds on targets, cells line etc…. Allow user to better compare biological results across publications (articles and Patents) and bioassays. Key component for patent where affinities are most of the time display as ranges and very difficult to compare. Insure end users to encompass all the affinity data that they are searching for without being an expert (knowing all the parameters and units used in publications) Facilitate analysis using third party tools (Spotfire, Pipeline Pilot) through the export. 29 HOW THE PX IS CALCULATED ? : AFFINITY RESULTS Results are expressed as –log10 (affinity) using pIC50, pEC50, pED50, pID50, pLC50, pLD50, pKi, pKd, pKb, pD2, pD’2, pA2 Æ pX = parameter Value Results are expressed as affinity using IC50, EC50, ED50, ID50, LC50, LD50, Ki, Kd, Kb, Ka, Ke ÆpX= -log10(parameter value) Remark If values are expressed in non molar units, they are first converted in M (using molecular weight, animal/tissue weight or volume) 30 HOW THE PX IS CALCULATED ? : % INHIBITION Results are expressed as % Inhibition - Concentration of the compound is not Available ÆpX is not calculated - Concentration of the compound is Available as : Æ pX is not calculated - RangesÆ - Single value Æ pX is calculated : - Assuming the compound could achieve a 100% inhibition -Assuming the slope (Hill coeff) = 1 -If %Inhibition > 25 ÆpX = -log10(IC50) - If %Inhibition < 25 ÆpX = 1 31





