ISPE DVC/NJ Seminar Facility of the Future Bruce Snyder Director, Process Automation
advertisement

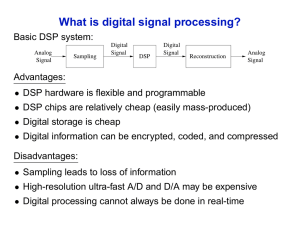
ISPE DVC/NJ Seminar Facility of the Future Bruce Snyder Director, Process Automation Integrated Project Services (IPS) 30 September 2015 Automation Considerations for Continuous Downstream Processing • • • • • • Business Drivers for Continuous DSP Processing Technical Enablers mAb Process and References Overall Automation Strategy Detailed Discussions Opportunities and Challenges Business Drivers for Continuous Downstream Processing • Increased bioreactor titers (2 g/L -> 10 g/L) drives very large tank sizes to handle batch volumes in DSP • Continuous DSP will reduce facility footprint and equipment sizes (~75% reduction) • Continuous DSP can be accomplished with fewer process steps • Faster process cycle times reduces WIP inventory and improves product stability • Continuous processing is widely used in other industries, but has been slow to be adopted by biotech Recent technological innovations bring us to the cusp of continuous DSP • Single use – fully disposable wetted paths eliminate CIP and SIP • Continuous single use centrifugation • Continuous multi column chromatography • Membrane Chromatography • Single pass tangential flow filtration mAb Process and References 50L SU BAG on WEIGH SCALE DOWNSTREAM PROCESSING 2000 L SUB 50 L 50 L CENTRIFUGATION 50 L DEPTH FILTRATION 50 L PRO A CMCC ACID/ BASE VIRAL INACTIVATION 50 L MEMBRANE CHROMATO GRAPHY 50 L 50 L CATION CMCC SPTFF • Brower, Mark; Hou, Ying; Pollard, David; Monoclonal Antibody Continuous Processing Enabled by Single Use; Continuous Processing in Pharmaceutical Processing; Wiley-VCH Verlag GmbH & Co., 2015. • Godawat et al; Periodic counter-current chromatography – design and operational considerations for integrated and continuous purification of proteins; Biotechnology Journal 2012. Overall Automation Strategy • Synchronization of unit operations, to maintain flow balance between unit operations • Each unit operation presents new, unique challenges – Flexibility in instrument and equipment setup • Continuous DSP will require a new approach for product release decisions – Process measurements, alarms and data collection to support real time product release decisions – How will boundaries for release decisions be defined? Synchronization of the DSP Unit Operations • Disturbance – unwanted increase/decrease in output flow – – • Bag fill weights will fluctuate to isolate other unit ops from the disturbance. Accomplished by High Weight Control Loop (setpoint 35 kg) and Low Weight Control Loop (setpoint 15 kg) Disruption – a stoppage in output flow of significant duration – – If a bag becomes filled or empty, flow rates at all unit operations must be adjusted to balance with the disrupted unit operation Detected by High Weight Alarm (45 kg) and Low Weight Alarm (5 kg) • Comparable schemes are used for level control in reflux drums and column bottoms in multi distillation column, continuous processing applications • A modern DCS is required to handle the complex requirements This diagram to be replaced with an accurate picture of the control scheme, to be drafted in Autocad Each Unit Op Presents New, Unique Challenges Protein A Chromatography Periodic Counter Current (PCC) from GE Healthcare features rapid cycling of small columns (3 to 9 at a time) Significantly higher column loading reduces media and buffer usage ΔUV method compensates for changes in feed stream composition and media performance Sequencing = f(target protein breakthrough) Challenges UV calibration (for dilute product concentrations, or high impurity loads) Must operate for 60 days without mechanical failure while maintaining low bioburden Continuous DSP will require a new approach for product release decisions Product release decisions may be periodic (ie daily) instead of gravimetric (ie batch quantity) • The process cell produces 2 products: medicine and data – – • New logic will be required to calculate start and stop times for queries into the continuous and event historians – – – • Rhythm wheel technique works as long as operating rates and hold up volumes remain constant Must compensate for flow rate measurement error, as it is compounded over time Must compensate for Non-plug flow and mixing in bags Opportunity to learn from discrete parts manufacturing control systems – – – – – – • The control system must generate a complete data set, including exception messages, which are relevant to the quantity of material presented for release Data must be “time shifted” to compensate for residence times, operating rates, and hold up volumes Divide the process stream into 100’s of “containers”, moving through the train on a “conveyour” Record the entry and exit times of each container at each unit operation Associate the alarm start and stop times with “last in” and “next out” container Calculate “conveyor speed” as a function of residence time, hold up volume, and operating rate Predict the arrival time of each container into the “output basket Generate the data and alarm messages for each output basket based on the time stamps of every container in the basket Continuous DSP will require a new approach for product release decisions • Process Analytic Technologies (PAT) will be required to measure all Critical Quality Attributes in line or at line. You may not achieve real time release without it. • PAT must be developed in conjunction with (at the same time as) process development and scale up. – Chances are regulatory approvals will be unobtainable if we try to “bolt it on” at full scale. • Develop a rational Process Validation Strategy which carefully considers CPPs and CQAs. – – • Multi Variate Data Analysis (MVDA) is a very powerful tool for process scale up and process control – • Robust process ranging studies at bench scale to develop the target envelope for each unit operation. It doesn’t have to be complicated to be powerful – – • If a parameter is well controlled, then it doesn’t have to be a Critical Quality Attribute Example – DNA clearance at polishing step Virus inactivation via pH treatment. Parameters are composition, contact time, temperature, mixing, flow rate, and mass balance. A MVDA model developed during process development can yield a well justified basis for controls and alarm limits to enable real time release. In Summary… • Single Use yields dramatic simplification in instrumentation, valves, and controls by eliminating CIP and SIP • However, Continuous Downstream Processing will drive increased automation complexity • Synchronization of the unit operations • New, unique challenges for controls at each unit operation • Process measurements, exception messages, alarms, and data collection to support product release • Process Analytical Technology • Continuous DSP will require a new approach for product release decisions • Process Analytic Technologies (PAT) will be required to measure all Critical Quality Attributes in line or at line. You may not achieve real time release without it. • PAT must be developed in conjunction with (at the same time as) process development and scale up. • • Develop a rational Process Validation Strategy which carefully considers CPPs and CQAs. • • • If a parameter is well controlled, then it doesn’t have to be a Critical Quality Attribute Example – DNA clearance at polishing step Multi Variate Data Analysis (MVDA) is a very powerful tool for process scale up and process control • • Chances are regulatory approvals will be unobtainable if we try to “bolt it on” at full scale. Robust process ranging studies at bench scale to develop the target envelope for each unit operation. It doesn’t have to be complicated to be powerful • • Virus inactivation via pH treatment. Parameters are composition, contact time, temperature, mixing, flow rate, and mass balance. A MVDA model developed during process development can yield a well justified basis for controls and alarm limits to enable real time release.