Photo-production of lactate from glyoxylate: how minerals can
advertisement
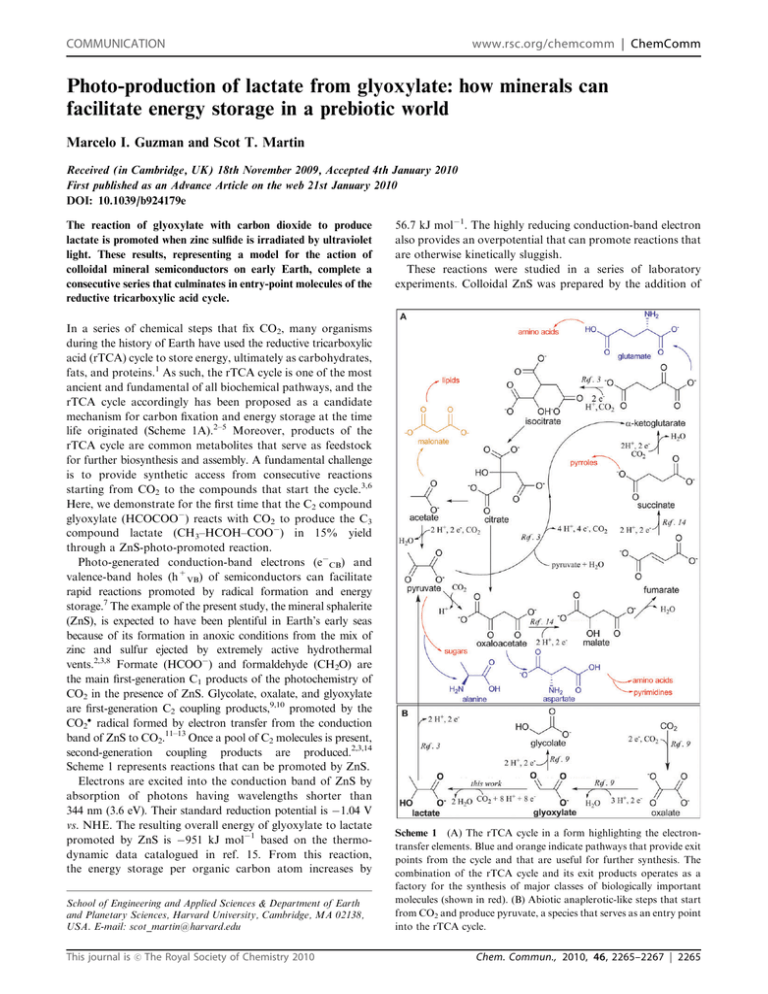
COMMUNICATION www.rsc.org/chemcomm | ChemComm Photo-production of lactate from glyoxylate: how minerals can facilitate energy storage in a prebiotic world Marcelo I. Guzman and Scot T. Martin Received (in Cambridge, UK) 18th November 2009, Accepted 4th January 2010 First published as an Advance Article on the web 21st January 2010 DOI: 10.1039/b924179e The reaction of glyoxylate with carbon dioxide to produce lactate is promoted when zinc sulfide is irradiated by ultraviolet light. These results, representing a model for the action of colloidal mineral semiconductors on early Earth, complete a consecutive series that culminates in entry-point molecules of the reductive tricarboxylic acid cycle. In a series of chemical steps that fix CO2, many organisms during the history of Earth have used the reductive tricarboxylic acid (rTCA) cycle to store energy, ultimately as carbohydrates, fats, and proteins.1 As such, the rTCA cycle is one of the most ancient and fundamental of all biochemical pathways, and the rTCA cycle accordingly has been proposed as a candidate mechanism for carbon fixation and energy storage at the time life originated (Scheme 1A).2–5 Moreover, products of the rTCA cycle are common metabolites that serve as feedstock for further biosynthesis and assembly. A fundamental challenge is to provide synthetic access from consecutive reactions starting from CO2 to the compounds that start the cycle.3,6 Here, we demonstrate for the first time that the C2 compound glyoxylate (HCOCOO ) reacts with CO2 to produce the C3 compound lactate (CH3–HCOH–COO ) in 15% yield through a ZnS-photo-promoted reaction. Photo-generated conduction-band electrons (e CB) and valence-band holes (h+VB) of semiconductors can facilitate rapid reactions promoted by radical formation and energy storage.7 The example of the present study, the mineral sphalerite (ZnS), is expected to have been plentiful in Earth’s early seas because of its formation in anoxic conditions from the mix of zinc and sulfur ejected by extremely active hydrothermal vents.2,3,8 Formate (HCOO ) and formaldehyde (CH2O) are the main first-generation C1 products of the photochemistry of CO2 in the presence of ZnS. Glycolate, oxalate, and glyoxylate are first-generation C2 coupling products,9,10 promoted by the CO2 radical formed by electron transfer from the conduction band of ZnS to CO2.11–13 Once a pool of C2 molecules is present, second-generation coupling products are produced.2,3,14 Scheme 1 represents reactions that can be promoted by ZnS. Electrons are excited into the conduction band of ZnS by absorption of photons having wavelengths shorter than 344 nm (3.6 eV). Their standard reduction potential is 1.04 V vs. NHE. The resulting overall energy of glyoxylate to lactate promoted by ZnS is 951 kJ mol 1 based on the thermodynamic data catalogued in ref. 15. From this reaction, the energy storage per organic carbon atom increases by School of Engineering and Applied Sciences & Department of Earth and Planetary Sciences, Harvard University, Cambridge, MA 02138, USA. E-mail: scot_martin@harvard.edu This journal is c The Royal Society of Chemistry 2010 56.7 kJ mol 1. The highly reducing conduction-band electron also provides an overpotential that can promote reactions that are otherwise kinetically sluggish. These reactions were studied in a series of laboratory experiments. Colloidal ZnS was prepared by the addition of Scheme 1 (A) The rTCA cycle in a form highlighting the electrontransfer elements. Blue and orange indicate pathways that provide exit points from the cycle and that are useful for further synthesis. The combination of the rTCA cycle and its exit products operates as a factory for the synthesis of major classes of biologically important molecules (shown in red). (B) Abiotic anaplerotic-like steps that start from CO2 and produce pyruvate, a species that serves as an entry point into the rTCA cycle. Chem. Commun., 2010, 46, 2265–2267 | 2265 O2-free Na2S (52 mM) to ZnSO4 (52 mM) with stirring under continuous argon bubbling. The size distribution and the structure of the colloidal particles prepared by this method were described previously.8 The 500 mL colloid was placed in a photochemical reactor and then augmented first with additional Na2S (1.8 mL; 2.22 M) and second with NaOOCHCO (18.2 mL; 48 mM). Carbon dioxide was bubbled continuously through the solution to decrease the pH from 12.3 to 7.5. The colloidal ZnS had a mass concentration of 2.3 g L 1, and the initial concentrations prior to irradiation were 1730 mM glyoxylate and 1760 mM sulfide available as HS . After sample preparation and under continuous CO2 bubbling, the mixture was irradiated at 15 1C under anoxic conditions for 2 h by an immersion medium-pressure photochemical lamp (Hanovia 7825). An intensity of 7.4 10 6 Einstein s 1 was determined by actinometry with KFe(C2O4)2.16 The reaction products as well as the remaining reactants were withdrawn and passed through a syringe filter (25 mm syringe filters, 0.2 mm pore size) to prepare for ion chromatography analysis (Dionex ICS-3000, equipped with an IonPac AS11-HC analytical column, suppression system, and a conductivity detector).3 The flow was set to 1.5 mL min 1 with a NaOH gradient, in which a mobile phase of 1.0 mM NaOH was used for 2 min followed by a linear increase of 2.16 mM min 1 up to 55.0 mM NaOH. The final concentration was held for 1 min. For some analyses, sulfate was removed by a 1 : 5 dilution of the supernatant (centrifugation for 5 min, 5000 rpm) in a 1 : 2 dilution with saturated barium hydroxide solution (i.e., totalling a 1 : 10 dilution). Lactate (1), glycolate (2), formate (3), glyoxylate (4), bicarbonate (5), sulfite (6), sulfate (7), oxalate (8), and thiosulfate (9) were quantified (Fig. 1). Although acetate (10) was separated from lactate using this method, it eluted simultaneously with glycolate. Thus, a new round of analyses using 1 mM NaOH with 10% methanol isocratic mobile phase at 1.5 mL min 1 was used to confirm that the reduction of glyoxylate stopped at glycolate and did not continue further to produce acetate (Fig. 2). In addition to ion chromatography, sulfide and pH were measured using ion-selective electrodes. A summary of results is given in Table 1 for the experiments and controls that demonstrate the heterogeneous photoproduction of the organic products. Measurements were carried out in the presence of one or more of glyoxylic acid (‘‘GA’’), colloid (‘‘ZnS’’), ultraviolet irradiation (‘‘hn’’; 200 to 400 nm), carbon dioxide (‘‘CO2’’), and sulfur-based hole scavenger (ST). The results summarized in Table 1 imply that the conditions Fig. 1 Chromatograms (A) before and (B) after irradiation. The number at the top of each peak corresponds to the labeling in the text. The inset shows an expanded region of chromatogram B. 2266 | Chem. Commun., 2010, 46, 2265–2267 Fig. 2 Chromatograms showing (A) glycolate and formate formed as products, (B) these products spiked with additional glycolate, and (C) these products spiked with acetate. The number at the top of each peak corresponds to the labeling in the text. Table 1 Experiments and controls to demonstrate that heterogeneous photoelectrochemistry is the production mechanism of lactate, glycolate, formate, and oxalate starting with glyoxylatea Productsc Conditions Experiment Control A Control B Control C Control D Control E GA ZnS hn CO2 ST LA Glyc FA OA + + + + + + + + + + + + + + + + + + + + + + b + + + + + + + + + + + + + + + a GA = glyoxylate, LA = lactate, Glyc = glycolate, FA = formate, and OA = oxalate. Table entries of ‘‘+’’ and ‘‘ ’’ indicate the presence or absence of a species, respectively. b [Na2S]0 o 10 3 mM. c Major products identified. for the formation of lactate (15% yield) require a mechanism that is driven by heterogeneous photoelectrochemistry. For the conditions of the full experiment (cf. Table 1), after 2 h of irradiation the initial glyoxylate concentration of 1730 mM decreased by 25% to 1290 mM. In addition to lactate, formate (1100 mM), oxalate (48 mM), and glycolate (18 mM) were also formed, as expected. Oxalate and glycolate were formed both as first-generation C2 coupling products of CO2 as well as by the oxidation and the reduction of glyoxylate, respectively. Further investigation to detect the best conditions for the formation of lactate are underway. Among other variables, the light intensity, the concentration of reactants, the pH value, and the temperature may affect the observed yield. For example, one experiment showed that the effect of dropping from 1760 to 570 mM initial sulfide was to decrease the yield of lactate from 15% to 4%. The explanation may be that stoichiometrically insufficient hole scavenger was present at the lower sulfide loading so that conduction-band electrons underwent recombination photophysics within the ZnS instead of interfacial electron transfer to form CO2 . As of yet, the full range of experimental conditions has not been investigated to determine the optimized yield, but the presented conditions of the experiment for 15% yield were designed as a scenario to resemble as closely as is presently known what were the conditions on early Earth. These new results can be combined with some already known facts, namely that (1) CO2 reacts in the presence of This journal is c The Royal Society of Chemistry 2010 tetramethylammonium chloride salt to produce C2 compounds including glyoxylate and (2) that lactate can be oxidized to pyruvate at high yield using ZnS photochemistry. Pyruvate is one entry point into the rTCA cycle (Scheme 1). Our present results that describe a pathway for the production of lactate from glyoxylate, taken together with what is already known, constitute a plausible production scheme on early Earth for the first metabolite of the rTCA cycle (i.e., through a series of cascading reactions starting from CO2). Our earlier work has examined the continuation from pyruvate to energy storage in other species of the rTCA cycle by the pathway of ZnSphoto-promoted reduction steps (Scheme 1).2,3,14 A direct mechanism thus emerges for the synthesis of useful energy-rich molecules through abiotic and nonenzymatic anaplerotic-like reactions in a prebiotic world. These results can be related to how life might have emerged on an early Earth composed only of simple chemical compounds.17 The scenario envisages a primary role for the mineral-based catalysis of processes. The minerals photocatalyze the primary steps of energy storage in small organic molecules. When immersed in sterile water with a suitable electron donor like HS and exposed to sunlight, sphalerite and other similarly reactive minerals conduct a train of reactions starting from CO2 to produce consecutively two-carbon, three-carbon, and longer chain compounds (Scheme 1). These steps have been demonstrated individually, and the next step in research would be a demonstration in toto. Also marked in Scheme 1 are more complex conversions observed for lactate and pyruvate, such as the one-step production of succinate (12% yield), a-ketoglutarate (50%), and isocitrate (11%). Complex conversions for other compounds include the reduction of oxaloacetate to malate (75%) and of fumarate to succinate (95%). The carboxylation of a-ketoglutarate to oxalosuccinate (2.5%) has also been demonstrated.2,3,14 These transformations combine to a generalized postulated model for carbon fixation promoted by photo-transformations on minerals. This hypothesized cycle was running prior to the appearance of enzymes and is evolutionarily linked in this way to present-day metabolism. This model, if supported in future studies by in toto operation of the cycle, would become an important achievement in linking the prebiotic and the living worlds. The science of the origins of life is divided into advocates of a replicator-first school and those who support a metabolismfirst vision.18 Both descriptions, however, require a supply of small molecules, either as building blocks for the replicator or as components for a primordial energy-driven self-sustaining metabolic cycle. Possible sources for this organic feedstock have been suggested, including atmospheric lighting-driven fixation in the Miller experiment,19 extraterrestrial infall,20 and mineral-catalyzed CO and CO2 fixation in hot springs and volcanos.21,22 The ZnS photochemical studies add an additional pathway to this group, one for which evidence continues to accumulate.2,3,8,14 The ZnS pathways can be applied as described to the rTCA cycle or alternatively in strong support of other proposed pathways, such as the glyoxylate scenario23 or the sugar model.24 All pathways may have been simultaneously operative on the early Earth. This journal is c The Royal Society of Chemistry 2010 Our research is summarized in Scheme 1 and apparent therein is that the formation of two rTCA cycle intermediates, cis-aconitate and citrate, have not been demonstrated by ZnS photoelectrochemistry. We have not yet investigated these pathways. Finding simple mechanisms for their production would advance the case for the chemical foundations of the rTCA cycle as being manufactured easily and routinely in early Earth’s oceans. Other pathways described in the literature have led to compounds of the rTCA cycle but only in low yield (o0.1%) and only for high temperatures (>500 K) and pressures (>50 MPa).21 For comparison, the hypothesized photo-driven mineral cycle integrates the use of ultraviolet energy, captured and transmitted through semiconductor minerals, to produce molecules that are otherwise inaccessible. The prospect that sphalerite can catalyze versatile and complex reactions all by itself is promising and possibly brings us much closer to understanding the chemical origins of life, supporting a view that life is bound to emerge because simple molecules central to metabolism can result and be driven in an energy-storing direction by carbon dioxide, light, and minerals. Support from the Harvard Origins of Life Initiative and NASA Grant NNX07AU97G issued through the Office of Space Science is gratefully acknowledged. Notes and references 1 V. Srinivasan and H. J. Morowitz, Biol. Bull. (Woods Hole, MA, U. S.), 2009, 216, 126. 2 M. I. Guzman and S. T. Martin, Int. J. Astrobiol., 2008, 7, 271. 3 M. I. Guzman and S. T. Martin, Astrobiology, 2009, 9, 833. 4 H. J. Morowitz, J. D. Kostelnik, J. Yang and G. D. Cody, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 7704. 5 G. Wachtershauser, Proc. Natl. Acad. Sci. U. S. A., 1990, 87, 200. 6 D. L. Nelson, M. Cox and M. Lehninger, Principles of Biochemistry, Worth Publishers, New York, 3rd edn, 2000. 7 M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69. 8 X. V. Zhang, S. P. Ellery, C. M. Friend, H. D. Holland, F. M. Michel, M. A. A. Schoonen and S. T. Martin, J. Photochem. Photobiol., A, 2007, 185, 301. 9 B. R. Eggins, P. K. J. Robertson, E. P. Murphy, E. Woods and J. T. S. Irvine, J. Photochem. Photobiol., A, 1998, 118, 31. 10 B. R. Eggins, P. K. J. Robertson, J. H. Stewart and E. Woods, J. Chem. Soc., Chem. Commun., 1993, 349. 11 B. R. Eggins, J. T. S. Irvine, E. P. Murphy and J. Grimshaw, J. Chem. Soc., Chem. Commun., 1988, 1123. 12 W. H. Koppenol and J. D. Rush, J. Phys. Chem., 1987, 91, 4429. 13 P. Neta, R. E. Huie and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 1027. 14 X. V. Zhang and S. T. Martin, J. Am. Chem. Soc., 2006, 128, 16032. 15 S. L. Miller and D. Smith-Magowan, J. Phys. Chem. Ref. Data, 1990, 19, 1049. 16 H. J. Kuhn, S. E. Braslavsky and R. Schmidt, Pure Appl. Chem., 2004, 76, 2105. 17 R. Shapiro, Q. Rev. Biol., 2006, 81, 105. 18 P. L. Luisi, The Emergence of Life: From Chemical Origins to Synthetic Biology, Cambridge University Press, Cambridge, 2006. 19 S. L. Miller, Cold Spring Harbor Symp. Quant. Biol., 1987, 52, 17. 20 C. Chyba and C. Sagan, Nature, 1992, 355, 125. 21 G. D. Cody, N. Z. Boctor, T. R. Filley, R. M. Hazen, J. H. Scott, A. Sharma and H. S. Yoder Jr, Science, 2000, 289, 1337. 22 G. Wachtershauser, Science, 2000, 289, 1307. 23 A. Eschenmoser, Tetrahedron, 2007, 63, 12821. 24 A. L. Weber, Origins Life Evol. Biosphere, 2001, 31, 71. Chem. Commun., 2010, 46, 2265–2267 | 2267



