Endocrine Disrupting Effects of Untreated and Ozone-treated Oil Sands in vivo
advertisement

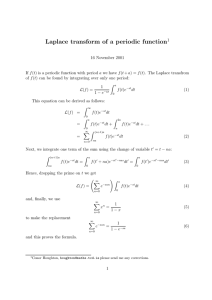
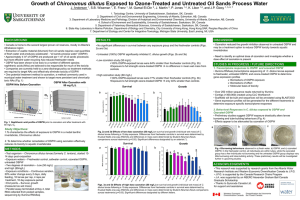
1 Endocrine Disrupting Effects of Untreated and Ozone-treated Oil Sands Process-affected Water (OSPW) in vivo and in vitro 1 Yuhe He1, Steve B. Wiseman1,, Nan Wang2, Leonidas A. Perez-Estrada2,3, Markus Hecker1,4, Jonathan W. Martin3, Mohamed Gamal El- Din2, John P. Giesy1,5,6,7 1Toxicology Centre, University of Saskatchewan, Saskatoon, SK, Canada. 2Department of Civil and Environmental Engineering, University of Alberta, Edmonton, AB, Canada. 3Division of Analytical and Environmental Toxicology, University of Alberta, Edmonton, AB, Canada. 4School of Environment and Sustainability, University of Saskatchewan, Saskatoon, SK, Canada. 5Department of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, SK, Canada. 6State Key Laboratory in Marine Pollution, Department of Biology and Chemistry, City University of Hong Kong, Hong Kong SAR, China. 7Department of Biology & Chemistry, City University of Hong Kong, Kowloon, Hong Kong, SAR, China. Background Results 1 – Effects on T & E2 Production in the H295R Cell Lines Large volumes of oil sands process-affected water (OSPW) are produced by the oil sands industry in Alberta, Canada. Overall more than 109 m3 of OSPW is stored on-site. Naphthenic acids (NAs) are a primary toxic organic constituent of OSPW. Ozonation decreases concentrations of NAs and attenuates some of the adverse effects of OSPW. • Exposure to OSPW and O3-OSPW caused significantly lesser T production and greater E2 production by H295R cells. • The 85% ozone treatment attenuated the effect on E2 production but not on T production. Objectives Investigate the endocrine disrupting effects of OSPW and ozone-treated OSPW in vivo and in vitro by quantifying: • Sex steroid hormone synthesis in the H295R cell line • Sex steroid receptor (AR & ER) signaling in the MDA-kb2 and T47D-kbluc cell lines • The transcriptional profile of hypothalamicpituitary-gonad-liver (HPGL) axis in adult male and female fathead minnows Figure 2: Fold change of T (left) and E2 (right) production by H295R cells exposed to control, OSPW and ozone-treated OSPW. Asterisks indicate significant differences from control (p < 0.05, one-way ANOVA with Dunnett’s tests). Results 2 – Effects on AR & ER Signaling in the MDA-kb2 and T47D-kbluc Cell Lines • Co-exposure to OSPW and O3-OSPW with T caused AR-mediated antiandrogenicity compared to T alone (showed as 1.0-fold). Methods • Exposure to OSPW and O3-OSPW caused an ER-mediated estrogenicity. OSPW • Ozone treatment attenuated the antiandrogenic but not the estrogenic effects. A Relative Response Relative Response Fresh OSPW was collected from the West In Pit during 2007 and 2010. Concentrations of NAs in all OSPW samples were around 19.7 mg/L as determined by HPLC/HRMS. B Figure 3: Anti-androgenic response in the MDA-kb2 cell line (left) and estrogenic response in the T47D-kbluc cell line (right). Different letters indicate significant differences between treatments (p < 0.05, one-way ANOVA with Tukey’s test). Results 3 – Effects on Gene Expression in HPGL Axis in Male & Female Fish Figure 1: Profile of NAs in OSPW (A) before and (B) after ozonation. Ozonation of OSPW decreased concentrations of parent NAs, and selectively targeted NAs with greater ring content and alkyl branching. Sex Steroid Hormone Synthesis H295R cell line • Concentrations of testosterone (T) & 17β-estradiol (E2) in medium were measured using ELISA. • OSPW was subjected to ozonation under two treatment conditions – 30 mg/L and 80 mg/L. Sex Steroid Hormone Receptor Signaling MDA-kb2 cell line • Anti-androgenic effects were determined by coexposure to OSPW and T. T47D-kbluc cell line • Estrogenic effects were determined by co-exposure to OSPW and ER antagonist (ICI). Samples tested in the receptor mediated assays were subjected to ozonation at a final concentration of 80 mg/L. HPGL Axis Transcriptional Profile Adult male and female Fathead minnows were exposed for 7-days under semi-static exposure conditions to the following solutions: • Freshwater control (facility water) • OSPW [NAs] = 19.7 mg/L • O3-OSPW [NAs] = 1.9 mg/L Quantitative real-time PCR was performed to measure abundances of transcripts of key genes along the HPGL axis. • Reference genes: 18S, RPL8 • Gonadotropins and receptors: KISS1R, GnRH2, GnRH3, GnRHR, FSHβ, FSHR, LHβ, LHR • Steroidogenesis: StAR, CYP11A, CYP17, CYP19a, CYP19b, 3βHSD, 17βHSD • Sex steroid receptors: AR, ERα, ERβ • Egg Yolk Proteins: VTG, CHG-L, CHG-H Figure 4: Abundances of transcripts of genes of the HPGL axis in adult male (upper panel) and female (lower panel) FHMs exposed to a freshwater control, OSPW, and ozone-treated OSPW. A) Brain, B) Gonad, C) Liver. Different letters indicate significant differences between treatments (p < 0.05, Kruskal-Wallis test). Male FHM • Greater abundance of KISS1R & CYP19B in the brain could stimulate FSHβ & LHβ and their receptors. • Greater abundance of CYP11A & 3βHSD in the gonads could result in increased steroidogenesis. • Greater abundances of ERα, VTG, CHG-L, & CHG-H in the liver indicated estrogenicity and anti-androgenicity. • Ozonation partially attenuated these effects. Female FHM • Greater abundance of LHβ but not FSHβ in the brain. • Lesser abundance of FSHR, LHR & CYP11A in the gonads might cause impaired synthesis of E2. • Lesser abundance of AR, ERα, ERβ, VTG, CHG-L & CHG-H in the liver might result from impaired synthesis of E2. • Ozonation did not attenuate most of these effects. Conclusion OSPW had endocrine disrupting effects on sex steroid synthesis, sex steroid receptor signalling, and expression of genes at all levels of HPGL axis in male and female FHMs. OSPW had different effects on male and female FHMs. Effects were more prominent in males than females. Ozonation attenuated effects of OSPW on certain endocrine endpoints. The results may provide a mechanistic basis for the assessment of impaired reproduction and less prominent secondary sexual characteristics by FHMs exposed to OSPW (Kavanagh et al., 2011, Aquat Toxicol 101, 214-20). Acknowledgements Research grant from the Alberta Water Research Institute to J.P.G, J.W.M and M.G.E.-D (Project # C4288) Discovery Grant from the Natural Science and Engineering Research Council of Canada (Project # 326415-07) and grants from Western Economic Diversification Canada (Project # 6578 and 6807) to J.P.G. Instrumentation grant from the Canada Foundation for Innovation (J.P.G.) NSERC Industrial Research Chair in Oil Sands tailings Water Treatment (M.G.E.-D.) Helmholtz-Alberta Initiative (M.G.E.-D. and J.W.M.) A NSERC Research Tools Program (M.G.E.-D). The authors wish to thank Warren Zubot of Syncrude Canada Inc. for supplying the OSPW. a