Theoretical Mobility Analysis of Ion Mobility Spectrometry Objective Methods
advertisement
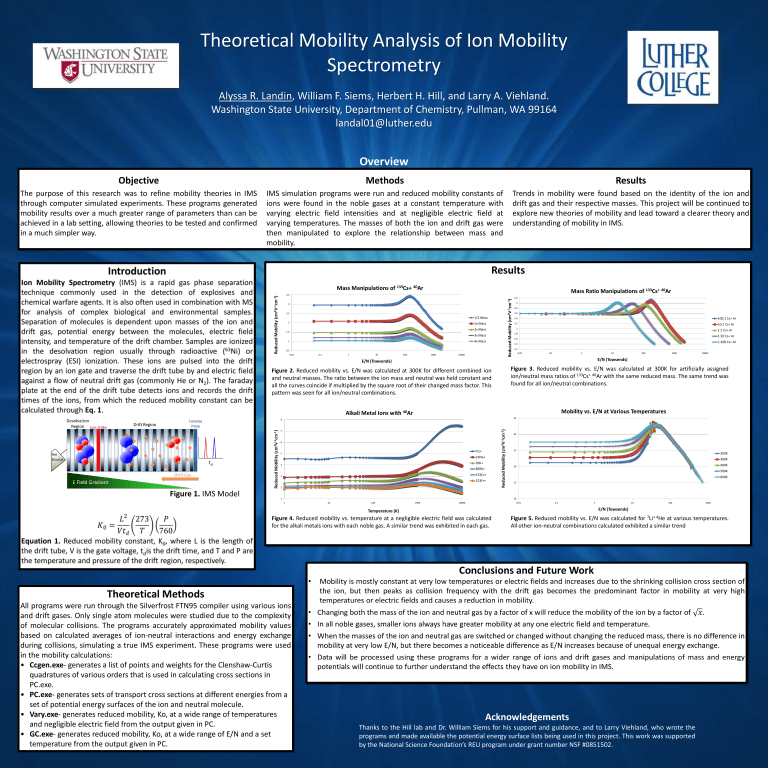
Theoretical Mobility Analysis of Ion Mobility Spectrometry Alyssa R. Landin, William F. Siems, Herbert H. Hill, and Larry A. Viehland. Washington State University, Department of Chemistry, Pullman, WA 99164 landal01@luther.edu Overview Objective Methods Results The purpose of this research was to refine mobility theories in IMS through computer simulated experiments. These programs generated mobility results over a much greater range of parameters than can be achieved in a lab setting, allowing theories to be tested and confirmed in a much simpler way. IMS simulation programs were run and reduced mobility constants of ions were found in the noble gases at a constant temperature with varying electric field intensities and at negligible electric field at varying temperatures. The masses of both the ion and drift gas were then manipulated to explore the relationship between mass and mobility. Trends in mobility were found based on the identity of the ion and drift gas and their respective masses. This project will be continued to explore new theories of mobility and lead toward a clearer theory and understanding of mobility in IMS. Results Mass Manipulations of 133Cs+ 40Ar Mass Ratio Manipulations of 133Cs+ 40Ar Reduced Mobility (cm2V-1cm-1) Reduced Mobility (cm2V-1cm-1) 3.5 3 2.5 1/2 Mass 2 1x Mass 2x Mass 1.5 3x Mass 4x Mass 1 0.5 0.01 0.1 1 10 100 1000 10000 3.2 3 2.8 2.6 2.4 100:1 Cs+ Ar 2.2 10:1 Cs+ Ar 2 1:1 Cs+ Ar 1.8 1:10 Cs+ Ar 1.6 1:100 Cs+ Ar 1.4 1.2 0.01 0.1 1 10 10000 Figure 3. Reduced mobility vs. E/N was calculated at 300K for artificially assigned ion/neutral mass ratios of 133Cs+ 40Ar with the same reduced mass. The same trend was found for all ion/neutral combinations. Figure 2. Reduced mobility vs. E/N was calculated at 300K for different combined ion and neutral masses. The ratio between the ion mass and neutral was held constant and all the curves coincide if multiplied by the square root of their changed mass factor. This pattern was seen for all ion/neutral combinations. Mobility vs. E/N at Various Temperatures Alkali Metal Ions with 40Ar 35 7 6 7Li+ 5 23Na+ 39K+ 4 85Rb+ 133Cs+ 3 223Fr+ 2 1 Reduced Mobility (cm2V-1cm-1) 8 Reduced Mobility (cm2V-1cm-1) 1000 E/N (Towsends) E/N (Towsends) Figure 1. IMS Model 100 30 25 200K 300K 400K 20 500K 600K 15 10 1 10 100 1000 10000 0.01 0.1 1 10 100 E/N (Towsends) Temperature (K) Figure 4. Reduced mobility vs. temperature at a negligible electric field was calculated for the alkali metals ions with each noble gas. A similar trend was exhibited in each gas. Figure 5. Reduced mobility vs. E/N was calculated for 7Li+ 4He at various temperatures. All other ion-neutral combinations calculated exhibited a similar trend Theoretical Methods All programs were run through the Silverfrost FTN95 compiler using various ions and drift gases. Only single atom molecules were studied due to the complexity of molecular collisions. The programs accurately approximated mobility values based on calculated averages of ion-neutral interactions and energy exchange during collisions, simulating a true IMS experiment. These programs were used in the mobility calculations: • Ccgen.exe- generates a list of points and weights for the Clenshaw-Curtis quadratures of various orders that is used in calculating cross sections in PC.exe. • PC.exe- generates sets of transport cross sections at different energies from a set of potential energy surfaces of the ion and neutral molecule. • Vary.exe- generates reduced mobility, Ko, at a wide range of temperatures and negligible electric field from the output given in PC. • GC.exe- generates reduced mobility, Ko, at a wide range of E/N and a set temperature from the output given in PC. 1000 Acknowledgements Thanks to the Hill lab and Dr. William Siems for his support and guidance, and to Larry Viehland, who wrote the programs and made available the potential energy surface lists being used in this project. This work was supported by the National Science Foundation’s REU program under grant number NSF #0851502.





