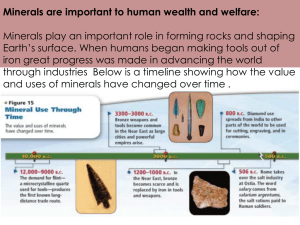
I T A K P E A N... C H A R A C T E R I... A N D
advertisement

Clay Minerals (1984) 19, 843-856 CHARACTERIZATION OF ITAKPE AND AGBAJA ( N I G E R I A N ) IRON ORES F. A. ADEDEJI AND REDUCIBILITY AND F . R . S A L E * Delta Steel Complex, Warri, Nigeria, and *Department of Metallurgy and Materials Science, University of Manchester, Grosvenor Street, Manchester M1 7HS (Received 18 January 1984; revised 28 June 1984) A B S T R A C T : Two iron ore samples from Nigeria have been examined using TG, DTA, EGA, XRD, and optical and electron microscopy. Itakpe iron ore is hematite-rich, this mineral being intergrown with magnetite, and silica is the major impurity. Agbaja ore is an acidic o61ite ore consisting of goethite and magnetite, with alumina, silica and phosphorus as major impurities. Itakpe is typical of a rich ore formed by magmatic segregation whilst Agbaja is a lean ore of sedimentary origin. Isothermal mass-change measurements in hydrogen and carbon monoxide in the range 800-1100~ show Agbaja to be less reducible than Itakpe; in particular, Agbaja is very irreducible at 1100~ because of sintering of the ore. Characterization and reducibility experiments were also carried out on Corby (Northamptonshire, UK) iron ore for comparison. Until recently, Nigeria has had no significant iron and steel industry. The total steel consumption in 1968 was estimated as 5.5 kg/caput, c o m p a r e d with 650 kg/caput for industrialized nations and a world average of 150 k g / c a p u t (Robson, 1968). This steel was obtained either by very small-scale internal manufacture or by large-scale importation. Iron ore reserves in Nigeria have been estimated at 11 000 million tonnes (Anon, 1963) and plans have been made to use these indigenous ores. The present study was designed to characterize two quite different major deposits from Itakpe and A g b a j a and to assess their suitability for exploitation. As preliminary work had shown the A g b a j a ore to be o f low grade, a low-grade U K ore from Corby, Northamptonshire, was studied for comparison, as this has been utilized over many years. MATERIALS AND METHODS General description o f ores The Itakpe sample was a compact, banded crystalline ore which varied in colour from grey to black. Much quartz veining was present. The crushed ore contained both reflective and dull particles, the latter being slightly magnetic. The A g b a j a sample consisted mainly of aggregates o f brown, compact, fine-grained material with some larger, extremely friable particles. This ore was strongly magnetic. The sample of C o r b y ore consisted mainly of reddish-yellow fine-grained material with some dispersed solid lumps. The ore was not significantly magnetic. 9 1984 The Mineralogical Society 844 F. A. Adedeji and F. R. Sale Chemical and mineralogical methods The lump ore samples were crushed mechanically and sieved to give particles in the size range 1-1.7 mm (16-10 mesh). Care was taken to ensure that this size fraction was representative of the lump material. The ore particles were crushed further for certain experimental techniques. Analysis for calcium, magnesium, iron, atuminium and manganese was made by atomic absorption spectrometry; silica was determined by a combination of gravimetric and colorimetric methods. X-ray diffraction analysis was performed using Cu-Ka radiation in a Siemens Kristalloflex 4 diffractometer. Samples were ground to 50-70 ~tm before mounting in thin layers on glass slides with collodion. For optical microscopy the samples were mounted in an acrylic medium and prepared in the conventional manner. A Cambridge 180 scanning electron microscope with a Kevex energy-dispersive detector was used for morphological studies and phase identification. A Stanton Redcroft automatically recording thermobalance (sensitivity + 0.1 mg) was used for thermogravimetric examination; samples, 0.2 + 0.02 g of 6 0 - 7 0 / z m material, were heated to 1100~ in air at a rate of 10~ min-L The ores were also studied by D T A over the temperature range 25-950~ using a heating rate of 10~ m i n - ' in both static air and in argon at a controlled flow rate of 100 ml min-~; 0.2 + 0.02 g samples were contained in Inconel crucibles (8 mm diameter • 10 mm high) with anhydrous AlzO 3 as the reference material. Evolved gas analysis was carried out using a VG Micromass 6 mass spectrometer fitted with a residual gas analysis source and a 60 mm, 90 ~ sector electromagnet. The resolving slit width of 0.5 mm gave a resolution of approximately 400 at mass 40. The gas was admitted to the mass spectrometer through a 0.5 mm diameter stainless steel capillary and calibrated glass leak. In operation, a 0.05 g sample was inserted into a silica vessel which was connected to the capillary and heated at 5~ min-' over the range 25-1000~ using a Stanton Redcroft linear temperature programmer. The m/e range 10-50 was scanned repetitively at intervals of 100 s. Reducibility experiments Isothermal mass-change determinations in the range 800-1100~ were performed on 0 . 1 - 0 . 2 g samples using either a glass-spring balance or a CI Electronics 2CT5 continuously recording electrobalance and both hydrogen and carbon monoxide atmospheres. Critical gas-flow rate determinations were carried out for both gases. Flow rates of approximately 200 ml min -1 and 150 ml min-' were used for both gases in the glass spring balance and electrobalance respectively. Fully-reduced and partially-reduced samples were retrieved from the balances for study. RESULTS Chemical analysis The results presented in Table 1 show that Itakpe ore is richer in iron than either the Agbaja or the Corby ores. Clearly, Itakpe is a fairly high-grade, acidic (or siliceous) ore, whereas Agbaja is a low-grade, high-phosphorus, acidic ore, Corby is also a low-grade, high-phosphorus ore which is basic (or calcareous) because of its high calcite content. Characterization of Nigerian iron ores 845 TABLE 1. Chemical analyses of the iron ores. Itakpe (Crushed) Element Itakpe (As received) wt% Fe Si A1 Ca Mg Mn P 59-10 6.80 0.38 0.36 0.028 0.007 nd nd Dark particles Light particles wt% 66.51 1-60 0-66 0.35 0.04 nd nd 5.04 40.40 nd nd 0.042 nd nd Agbaja wt% Corby wt% 54.17 4.48 5.25 0.50 0.08 0-083 2.69 37-85 6.42 1.75 8.76 1.32 0.40 1.12 not detectable, X-ray diffraction analysis Itakpe ore was shown to consist mainly of hematite with a detectable amount of magnetite, a significant amount of quartz, and a trace of corundum. In the Agbaja ore, the minerals identified were magnetite, goethite, corundum and quartz, with traces of iron and aluminium phosphates. The Corby ore gave a more complex diffraction pattern corresponding to the presence of siderite, calcite, dolomite and the two phosphate minerals vivianite and apatite. Thermogravimetry T G results for the three ores are presented in Fig. 1. The ltakpe ore showed a slight weight gain of ~ 1%, mainly above 850 ~ C, caused by oxidation of the magnetite present in | +2 0 -2 Itakpr % wt -6 Change -10 -14 -18 -22 I 200 . I I 400 600 Temperature ~ FIG. 1. TG data for the three ores. I 800 F. A. Adedeji and F. R. Sale 846 the ore. The Agbaja ore lost approximately 7% weight over the temperature range 50-500~ with a rapid weight loss occurring between 250~ and 500~ due to dehydroxylation of goethite. Above 900~ an increase in weight caused by the oxidation of magnetite is evident. The Corby ore showed three separate weight losses at 180-380~ 450-650~ and 700-830~ in addition to a small loss that occurred between room temperature and 160~ The total weight loss of this ore was ~22%. Differential thermal analys& The DTA curve for the Itakpe ore (Fig. 2) showed endotherms, possibly associated with quartz and silicates, with peaks (and extrapolated onsets) at 580~ (575~ and 890~ (870~ and an exotherm at 910~ which coincided with the weight gain detected as a result of oxidation. The Agbaja ore showed three endothermic peaks, the latter two of which coincided with those of the Itakpe ore and were presumably also associated with quartz and silicates. However, a lower-temperature endotherm with a peak at 340~ and an extrapolated onset at 250~ appears on the DTA curve of this ore. An exotherm, similar to, and of the same origin as, that caused by the oxidation of the Itakpe ore at approximately 910 ~C, was also detected for the Agbaja ore. Evolved gas analysis Figs 3 and 4 show the evolved gas analysis data obtained for the Agbaja and Corby ores respectively. The curves shown for water vapour and carbon dioxide correspond to peak heights observed at m/e values of 18 and 44 respectively, after correction for background. Itakpe ore did not evolve any gas during heating. A relatively large amount of water vapour was evolved on heating the Agbaja ore from room temperature to 600~ The rate of evolution increased markedly from 245 ~ peaking at 340 ~C, and corresponding to the first peak on the DTA curve for this ore. No other gases were detected during heating the I __ I I I I 340 U 1- l ! I 890 f~O Itakpr "6 ,,Q C W ~580 ! 200 I I 400 I l 600 Temperatureoc ~0 890 I I 800 FIG. 2. DTA trace for ]takpe and Agbaja ores. I Characterization of Nigerian iron ores 847 EGA Agbaja 40 Peak Height (arbY u.it$) 20 O 200 400 600 Temperature~ FIG. 3. EGA data for Agbaja ore. EGA Corby i;co2 9 40 Peak Height _ / (orbYunlts) / 20- -/ / ................/ I 0 200 400 600 Temperature ~ ' I il %. I .%%! 800 FIG.4. EGA data for Corby ore. Agbaja ore. The Corby ore gave two major peaks at 500~ and 750~ and one minor peak at 630 ~C for carbon dioxide and one peak at approximately 300 ~C for water vapour. A small secondary evolution of water vapour occurred at ~475 ~ C. Microscopic examination Three distinct phases were identified in the Itakpe ore by optical microscopy, these appearing as grey, white and mottled white/black areas in the micrograph in Fig. 5. Examination by scanning electron microscopy/energy-dispersive analysis showed that the grey phase was quartz, the white phase hematite, and the mottled areas intergrowths of hematite and magnetite. The Agbaja ore showed a pisolitic texture in polished section (Fig. 6). The iron was concentrated in the pisolites whereas the major impurities were present in the matrix. The Corby ore showed a multi-phase o61itic texture (Fig. 7). F. A. Adedeji and F. R. Sale 848 FIG. 5. Optical photomicrograph of Itakpe ore. FIG. 6. Optical photomicrograph of Agbaja ore. FIG. 7. Optical photomicrograph of Corby ore. Kinetics of reduction of ores Hydrogen reduction. Two of the three ores were heat-treated before reduction to remove the volatile components and so permit a true comparison of reducibilities, as determined by mass loss. The isothermal mass-change data obtained at 800, 900, 1000 and 1100~ (Figs 8-10) show that for all ores the rate of reduction increased with increase in temperature, except for reduction at 1100~ For both Itakpe and C o r b y ores the rates of reduction were in the order IO00~ ) 1100~ ) 9 0 0 ~ >> 800~ whereas for the Agbaja ore they were in the order 1 0 0 0 ~ 1 7 6 1 7 6 1 7 6 The rapidity with which hydrogen acts as a reducing agent for all the ores is evident in Figs 8-10, as almost all the curves approach maximum mass loss within the first fifteen minutes of reduction. 849 Characterization of Nigerian iron ores Ti me O (minutes) ,o, • ~?, ~o i 7 • I IOO°C %wt L°s~4 21 28, l , I , Itakpe I Ha Red"" FIG. 8. Isothermal mass-change data for H 2 reduction of Itakpe ore. Ti me IO ix 20 ' I~\ (minutes) 30 ' 7L\\\ O/oW~I \ \ \ ' ~ . ~ ,,o;o~1 , 9oo:c l " "°°Cl L°ssI4 %%\a 2 • o IOOO°C J e ~ e ~ e g L ~ Agbaja H 2 Red TM F]G. 9. Isothermal mass-change data for H 2reduction of Agbaja ore. F r o m the d a t a it is apparent that Itakpe ore is the most easily reduced at 800, 900 and 1000°C; C o r b y ore is the least reducible at these temperatures but at 1100°C A g b a j a is the least reducible of the ores. Carbon monoxide reduction. The deposition o f carbon by the disproportionation of carbon monoxide caused difficulties in interpreting the results at 800 and 9 0 0 ° C . F. A. Adedeji and F. R. Sale 850 Ti me (minutes) I0 20 0 30 O]o Wt 7 ,, Z2 "~'~l~O.~" 21 I I O0~ I000 oC 900 ~ C BOO ~ C Corby H 2 Red"" FIG. l 0. Isothermal mass change data for H 2reduction of Corby ore. Consequently, most experimental effort was concentrated on reduction at 1000 and 1100~ Not only was carbon deposition minimized at these temperatures but, at the same temperatures, the hydrogen reduction experiments showed interesting effects. For both the Itakpe and Corby ores the rate of reduction at 1000 and 1100~ (Fig. 11) increased with increase in temperature. However, as in the hydrogen reduction experiments, the rate of reduction of Agbaja ore decreased significantly with increase in temperature. Fig. 11 Ti me 60 0 I % Wt Loss (minutes) 120 I Joo~ \ ~ .......u..o...o.~ I o Iooo c \~"..i ........ 14 180 I ~coo~ _...--. -r6~oc \ .\..~ 2B " ._.~ I IOO~ i\ O --. .I . 60 Itakpe Agbaja Corby ...................... I 120 Reduction 1 180 in CO FIG. 11. Isothermal mass-change data for CO reduction of three ores at 1000 and 1100~ Characterization of Nigerian iron ores 851 also shows that appreciable carbon deposition occurred with the Itakpe ore at 1000~ whereas no detectable deposition occurred for the other ores. The absence of carbon deposition with the lean ores agrees with earlier observations (Bones et al., 1938; Soma, 1963) that the rates of carbon deposition are lower with lean ores than with rich ores. Such observations are explained by the fact that freshly produced iron catalyses the disproportionation of carbon monoxide. From Fig. 11 it is obvious that Itakpe is the most reducible ore at both temperatures, the sequence of reducibility being Itakpe > Agbaja > Corby at 1000~ and Itakpe > Corby > Agbaja at 1100~ Microscopy of reduced ores Samples of the three ores, partially and fully-reduced in both hydrogen and carbon monoxide, were examined by optical and electron-optical techniques. Scanning electron micrographs of the product of full reduction of Itakpe ore with carbon monoxide at 1000~ and 1100~ (Figs 12a,b) show no evidence of the carbon deposition which was detected at 1000~ by the isothermal mass-change measurements. The most significant feature observable is the slight sintering and closing-up of pores that occurs at 1100~ The morphologies of the products obtained by hydrogen reduction were similar to those obtained in carbon monoxide although the products from hydrogen reduction were more fully sintered at all temperatures than those obtained in carbon monoxide. Both lower-grade ores gave spongy, although very impure, iron products with many features similar to those in Fig. 12. However, the products from Agbaja ore were always more fully sintered than those from the Corby ore. Fig. 13 shows an optical micrograph of Agbaja ore reduced at 900~ in hydrogen. The pisolitic structure of the original ore is still present; however, the lighter phase is now metallic iron which, at higher magnifications, can be seen as individual grains some 5/am in diameter (Fig. 14a). The topochemical nature of the reduction process was clearly seen in all partially-reduced samples. Thus in a sample of Agbaja ore partially-reduced in hydrogen at 1000~ (Fig. 14b), iron rims encapsulate the remaining unreduced oxide (in this case wustite). In an attempt to explain the large decrease in reducibility that occurred with the Agbaja FIG. 12. Scanning electron micrographsof Itakpe ore reduced in carbon monoxide at (a) 1000~ and (b) 1100~ 852 F. A. Adedeji and F. R. Sale FIG. 13. Optical micrographs of Agbaja ore reduced in hydrogen at 900~ FIG. 14. Optical micrographs of Agbaja ore reduced in hydrogen at 1000~ (a) fully reduced, (b) partially reduced. FIG. 15. Scanning electron micrographs of Agbaja ore after heat treatment at 1IO0~ ore at l l 0 0 ~ fully-reduced samples and samples heated to l l 0 0 ~ in argon were examined. The fully-reduced samples were found to contain massively densified iron, but, even more significantly, the ore heated in argon had densified considerably by the sintering together of the individual particles (Fig. 15). Neither of the other ores showed this effect. Characterization of Nigerian iron ores 853 DISCUSSION Characterization of ores The Itakpe ore is a fairly high-grade, dense iron ore consisting of ~60% iron with silica as the major impurity. There is no evidence of significant quantities of other deleterious elements and the absence of phosphorus is of particular importance. Thermoanalytical data indicate the absence of volatile materials. As indicated in Table 1, much of the silica content of the ore can be removed following simple mechanical crushing, the iron content of a 'hand-picked' concentrate being ~66%. From the optical studies of polished sections, XRD examination and SEM/energy-dispersive analysis, the mineral assemblage of the Itakpe ore comprises hematite, magnetite and quartz. The hematite is specular and where hematite and magnetite co-exist they are intimately intergrown, possibly as a result of martitization (Mihelik & Smits, 1973; Harbord & Goldring, 1965). Because of its grade and mineral composition, Itakpe ore compares favourably with the rich ore from the Kiruna district of Sweden (Percival, 1955; Parks & McDiarmid, 1970) and the Itabirite type ore from Mina Gerias, Brazil (Pounds, 1959). The characterization studies suggest that, since the major portion of the quartz can be liberated from the Itakpe ore by simple mechanical crushing, beneficiation could be achieved by crushing followed by magnetic or electrostatic separation or riffle tabling. Such separation would yield a product suitable for 'direct-reduction' in a non-conventional iron-making process (such as a rotary kiln) for the production of sponge iron. The Agbaja ore is a fairly lean, acidic iron ore with a high phosphorus content. The ore is an earthy, friable material containing magnetite and goethite, together with minor aluminosilicates and phosphates of iron and aluminium. The ore contains approximately 54% iron and shows thermal effects associated with the elimination of water. The major water peak detected by EGA at 340~ (Fig. 3), which corresponds to the broad DTA endotherm at 340~ (Fig. 2) and the mass change observed at approximately this temperature by TG, is probably caused by the dehydroxylation of goethite. Optical and scanning electron microscopy both show the pisolitic/o61itic nature of this ore, which is typical of many sedimentary iron ores (Parks & McDiarmid, 1970). O61itic ores vary considerably, both with respect to amount and composition of the o61iths, which may consist of hematite, goethite, siderite or chamosite. The texture and chemical composition of the Agbaja ore suggests that despite its magnetic character it cannot be easily beneficiated for use in a direct, non-conventional ironmaking process. The various slag-forming constituents (silica, alumina, lime and magnesium oxide) are so closely associated with the iron-bearing constituents that separation is impossible to achieve by simple physical means. Furthermore, the high phosphorus content (about 2.5%) would probably give rise to problems in steel production unless a conventional, oxidizing, liquid-metal process (such as basic oxygen steelmaking) is used following blast-furnace production of liquid iron. Although the Corby ore sample studied here is lean (approximately 37% iron) it is much richer than present-day ores from the Northamptonshire fields which contain less than 23% iron and are now used more as a source of limestone than iron. The analytical data given in Table 1 closely resemble those found in the literature (Pounds, 1959; Reeve, 1948; Wild & Saunders, 1950). The ore is more basic and contains less phosphorus than the Agbaja ore. The major iron-bearing mineral is siderite which, with calcite and dolomite, is embedded in 854 F . A . Adedeji and F. R. Sale a matrix of alumina, silica and phosphates. The ore samples are friable and consist of o61iths of siderite varying in shape from spheroidal to ellipsoidal. The Corby ore is confirmed as a carbonate ore by the thermo-analytical data which show three stages of evolution of carbon dioxide--at 500~ from siderite and at 625~ and 750~ from dolomite and calcite. The small water evolution detected for this ore is probably associated with adsorbed moisture and some unidentified hydrated mineral. From the foregoing discussion it is clear that there are textural similarities between the Agbaja and Corby ores, although in the former the iron is present mainly as an oxide/hydroxide and in the latter as carbonate. Neither ore can be up-graded sufficiently for use in a direct ironmaking process and so both should be processed in the blast furnace. Corby ore has been economically treated by making it into sinter for the blast furnace (Harbord & Goldring, 1965; Fitton & Goldring, 1966; McBriar et al., 1954). However, this ore has the advantage over the Agbaja ore of being almost self-fluxing. Thus the undesirable silica and alumina combine with the lime and magnesia to produce a readily fusible slag. The basicity value calculated for the slag-forming constituents of the Corby ore is approximately 0.9, a value almost equal to some used in regular blast furnace practice. Consequently, this ore requires only a small amount of lime addition in use. For the Agbaja ore the basicity value is very low (approximately 0.035) and hence the ore would need significant additions of lime, limestone or a lime-rich ore to make a self-fluxing sinter or pellet suitable for iron production. The basicity value of the Itakpe ore is approximately 0.025 and, if this were to be used in a blast furnace process, it would require limestone loadings, but to a smaller extent than the Agbaja ore as the total amount of silica is low. The reducibilities of the ores The results presented previously indicated that the Itakpe ore is the most reducible of the ores studied. The rapid reduction of this ore can be explained on the basis of its purity and the presence of hematite, which has been shown to be the most reducible of the iron oxides (Bitsaine & Joseph, 1955; Brill-Edwards & Samuel, 1965; Edstrom, 1967). The resistance to reduction of Agbaja and, to a large extent, of Corby ores can be attributed to the presence of large quantities of impurities and gangue, which reduce the access of reductant to the iron-containing phases, as well as to the actual iron-bearing phases available for reduction (Bitsaine & Joseph, 1955; Elgeassy et al., 1977). The reduction curves (Figs 8-11) closely resemble those given in the literature for various similar studies and can be explained in terms of the mass-transfer processes involved in reduction. Initially, rapid reduction occurs because of the easy access of the reducing gas to the ore. The subsequent decrease in rate is attributable to either of two major contributing factors--namely reduction in the surface area of remaining unreduced ore and the decreased rate of diffusion of reductant to the ore surface caused by a build-up of a product layer and unreduced material as the reaction proceeds. The initial, almost linear, rate of reduction, especially for dense iron oxides, has been attributed to a phase-boundary controlled reaction which produces a topochemical type of reaction (McKewan, 1962; Shehata & Ezz, 1973; Viswanath et al., 1977; English & Roberts, 1978). Further decreases in reaction rate may arise from sintering and recrystallization of porous iron or sintering of the unreduced ore or gangue materials. At high temperatures, sintering of the iron product has been shown to affect greatly the reduction rate of a variety C h a r a c t e r i z a t i o n o f N i g e r i a n iron ores 855 of iron ores and oxides (Edstrom, 1957; Cox & Sale, 1974; Lien et al., 1971; Gray & Henderson, 1966). Morrison et al. (1978) explained the limitation of reduction in their studies by the sintering of the reaction products. From the isothermal mass-change data (Figs 8-10), and from the microstructural studies, it is apparent that at 1100~ sintering of the product causes diffusion to become the rate-controlling step. However, the results in Fig. 11 indicate that sintering is not so extensive during reduction with carbon monoxide, possibly because of carbon deposition. Thus, although the overall reduction rates with carbon monoxide are generally lower than with hydrogen (certainly at lower temperatures), the lower level of sintering of the iron product in carbon monoxide allows an increase in temperature from 1000~ to 1100~ to bring about an increase in reduction rate for both Itakpe and C o r b y ores. The reducibility of Agbaja ore, however, decreases in both hydrogen and carbon monoxide as the temperature is increased from 1000 to 1100 ~ C because of sintering of the ore particles. CONCLUSIONS 1. Itakpe ore is a rich hematite ore in which some hematite forms an intergrowth with magnetite. The main impurity is silica. Following liberation by mechanical crushing and physical separation of the quartz, the Itakpe ore is suitable as a feedstock to one of the direct reduction methods of ironmaking. The ore is typical of one formed by magmatic segragation. 2. Agbaja ore is an acidic pisolitic/o61itic ore consisting of goethite, magnetite and major amounts of aluminous and siliceous minerals. It cannot be used directly in a blast furnace or other reduction process without further treatment, e.g. sintering, pelletizing or briquetting. Corby ore is confirmed as a basic o61itic carbonate ore with a basicity of ~0.9. Both Agbaja and Corby ores are lean and of sedimentary origin. 3. Reduction studies of the three ores indicate that Itakpe ore is the most reducible in both hydrogen and carbon monoxide, whereas Corby ore is the least reducible within the temperature range 8 0 0 - 1 0 0 0 ~ At 1100~ Agbaja ore is the least reducible because sintering of the ore prevents access of the reducing agent. ACKNOWLEDGMENTS The authors thank the OYO State Government of Nigeria for maintenance funding for one of the authors (FAA) and to the Federal Ministry of Mines and Power in Lagos, Nigeria, for the provision of the Itakpe and Agbaja ore samples. Thanks are also due to the University of Manchester for the provision of laboratory facilities. REFERENCES ANON(1963) Steel Review 30, The British Iron and Steel Federation Quarterly. BITSAINEG. & JOSEPHT.L. (1955) J. Metals 177, 639. BONESW.A., SAUNDERSH.L. & TRESS,H.J. (1938)J. Iron and Steel Inst. 1, 85. BRILL-EDWARDSH. & SAMUELR.L. (1965) J. Iron and Steel Inst. 203, 361. Cox T.G. & SALEF.R. (1974) Ironmaking and Steelmaking 4, 234. EDSTROMJ.O. (1957) Jernkont. Ann. 141,809. ELGEASSYA.A., SHEHATAK.A. & EZZ J.Y. (1977) Iron and Steel Int. 5, 331. ENGLISHB.L. & ROBERTD.A. (1978) Trans. Inst. Min. Metall., Sect. C 87, 113. FITTONJ.T. & GOLDRINGD.C. (1966) J. Iron and Steel Inst. 204, 452. GRAYN.B. & HENDERSONJ. (1966) Trans. Met. Soe. AIME 236, 1213. 856 F, A. Adedeji and F. R. Sale HARBORD N.H. & GOLDRINGD.C. (1965) J. Iron and Steel Inst. 203, 349 LIEN H.O., EL MEHAIRYA. & ROSS H.U. (1971) J. Iron and Steel Inst. 209, 341. McBR1AR E.M., JOHNSON W., ANDREWSK.W. & DAVIESW. (1954)./. Iron andSteellnst. 177, 316. McEWAN W.M. (1962) Trans. Met. Soe. AIME 224, 387. MIHELIK P. & SMITS, G. (1973)NIMReport 1565, Johannesburg, S. Africa. MORRISON A.L., WRIGHT J.K. & BOWE1NGM.MCG. (1978) Ironmaking and Steelmaking 5, 32. PARKS C.J. & MCDIARMIDR.A. (1970) Ore Deposits, p. 245. W.H. Freeman Ltd. POUNDS N.J. (1959) The Geography of Iron and Steel, p. 11. Hutchinson and Co. Ltd. REEVE L. (1948)J. Iron and Steel lnst. 159, 277. ROBSON R. (1968) ECA Paper A4 in: 2ncl Int. Symp. on the Iron and Steel Industry. UNIDO, Moscow. SHEHATA K.A. & Ezz, S.Y. (1973) Trans. Inst. Min. MetalL Sect. C 82, 38. SOMAT. (1963) J. Iron and Steel Inst. Japan 49, 1645. VlSWANATHR.P., VISWANAT. B. & SASHTRIM.C. (1977) Trans. Japan Inst. Metals 18, 149. WILD R. & SAUNDERSH.L. (1950) J. Iron andSteellnst. 173, 198.