Notes for Week 3.
advertisement

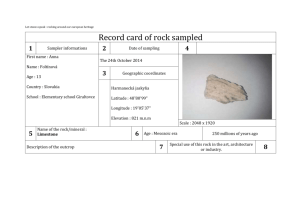
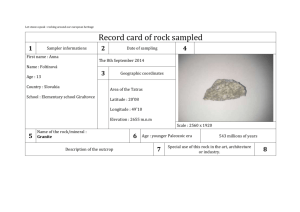
Notes for Week 3. The chapter on the Moon discusses its gross features (gross=big scale in this context), gives some background on minerology and rock formation, talks about how ages for rocks are derived, and then describes crater-forming processes. There is a lot of vocabulary, and the words are those you can hear the astronauts using in films of the lunar landings. Some amplification and comments: The concept of radiative equilibrium temperature is very important. Here is the basic derivation of this important quantity. Consider a spherical planet a distance d from a star of luminosity L. The fraction of L that this planet intercepts is (cross-section of the planet)/(area of sphere of radius d) = πr2 /4πd2 Of the intercepted radiation, some fraction A will be reflected. For bodies in our solar system, most of the solar radition is in the visible, so we consider an average value in the visual: Avis. A perfect blackbody planet at a uniform temperature T will radiate energy away at the rate: LPlanet = 4πr2 σ T4 . A basic property of blackbodies is that they emit radiation with the same efficiency that they absorb it. The planet will radiate most of its energy in the infrared [a statement we can check after finishing the calculation of T] so fraction absorbed = 1-fraction reflected = 1-Avis and efficiency of re-radiation = (1-AIR) This gives (1-A vis ) (πr 2 /4πd 2 ) L = (1-A IR ) (4πr 2 ) ( T e q 4 ) as the fundamental equation for the radiative equilibrium temperature of a planet. You should be able to answer these questions: Why is it called "equilibrium" temperature? Hint: What would happen if the planet were hotter than or colder than Teq ? What would the equation look like if the Earth, instead of being round, were a cube with one face perpendicular to the Sun-Earth line? As we found with LC5, the above determines the averge value of T4 over the surface of the planet; the local value may be quite different. Let's calculate the radiative equilibrium temperature of a surface oriented at an angle θ to the line to the Sun: perpendicular θ surface to the Sun For this situation: (1-A vis ) (C/4πd 2 ) L = (1-A IR ) (A) ( T e q 4 ) where C = cross-sectional area in the beam of sunlight and A = surface area radiating. We'll assume the surface is able to radiate only from the topside (as it is sitting on an insulating layer of soil, for example). Then C/A = cos θ and ( T e q 4 ) = [(1-A vis )/(1-A IR )] [(Lcos / 4πd 2 )] The biggest factor making the summer warmer than the winter is this one: the Sun's altitude* in the sky is greater at a given time of day in summer than in winter. Second biggest factor is the length of the day - more hours of sunlight in the summer. The third biggest factor is how much of the atmosphere the sunlight has to traverse as it heads for the ground - more in the winter when the angle is lower, so more light is scattered out of the path, so less reaches the ground. Why is the distance from the Earth to the Sun NOT an important factor? _____________________________________________________________________ The second big idea in this chapter is the description of how the melting and solidification process works, and how this information can be used to deduce how deep and how hot a rock was when it was melted. Key terms: eutectic temperature; eutectic composition ternary phase diagram Consider first a mixture of two minerals. Figure 3-12 illustrates how the solid, partially melted, and melted phases for this substance depend on the temperature and the % of each material present. Consider each of the following three possibilities: (a) you have a sample of pure Pyroxene. As you heat the rock, it melts at what T? (b) you have a sample of pure Plagioclase. As you heat the rock, it melts at what T? (c) you have a mixture that is about 45% plagioclase, 55% pyroxene. It melts at what T? * Altitude = angle from the horizon to the object, measured vertically. The zenith angle, θ in our equation, is (90°-altitude) in degrees or (π/2 -altitude) in radians. (d) you take a bunch of pure pyroxene rock and grind it up; you mix this with a similar amount of pure pyroxene. As this is heated, what happens? What would happen if you used mostly one and only a little of the other? A familiar case of 2 substances: Sprinkle salt crystals onto an ice cube. What happens? Consider next a mixture of 3 minerals - figure 3.13. If we start from liquid and let it solidify, some portions will solidify first, leaving a changed composition of the liquid portion. The path that the liquid portion will take in this diagram depends on the properties of the minerals and on the pressure. Thus, the final rock will give evidence of the conditions under which it solidified - small crystals for rapid solidification, big ones for slow solidification. More of some components for a rock formed under higher pressure than under lower pressure. A familiar case of a mixture of three substances: Consider ice on a gravel road on a very cold day, with added crystals of salt. What happens as it starts to melt? Altogether, Moon rocks reveal: (a) ongoing "processing" of lunar material by impacts. (b) evidence for widespread (liquid) magma on the Moon soon after it formed. _____________________________________________________________________ The third big idea is the use of radioactively unstable isotopes to date a rock. For an unstable isotope U that decays forming (among other things) a product D: dN U/dt = - λ t so N(t) = NoU e- λ t while ND(t) = NoD + NoU (1-e- λ t) If we know NoD then we can simply look at the ratio NU/ND = e- λ t /(1- e- λ t ) to determine t. This might be the case for example if D is something that would not normally be part of the rock (or the particular mineral in the rock) but that is trapped in it if it is produced there by radioactive decay after the rock forms. Also, if D is one isotope of an element that has other isotopes, then we can compare the ratio of the isotopes of D to what one finds where D is present without any U. Another version is to look for variations within your sample in the ratios U/D and D/D', where D' is the other isotope of D. This is the basis for the analysis on page 54. The analysis on page 54 assumes that the isotope ratio is constant while the ratios of Rb to Sr depend on the exact conditions where the mineral grains formed. This is a good assumption. If you start with ingredients with a uniform isotope ratio and form a variety of minerals, the minerals (and resulting rocks) will have essentially the same isotope ratio. Why? Well - two isotopes of one element have the same chemical properties. They differ only in atomic mass. To separate isotopes is pretty hard; that was the main assignment for the Ames Laboratory here during WWII. What is meant by the "age" of a rock, measured this way? It is the time since the rock was last melted. The oldest rocks on Earth are < 4 Gyr old, measured by this method; does that mean the planet is <4 Gyr old? A few things to think about in preparation for exam I: We have covered Chapter One: Topics related to Earth Figuring out the arrangement and scale of the Solar System Geometry of measuring planet orbits in AU Techniques for determining the AU in km Figuring out the origin of the Earth - processes that modify its surface. Chapter Two: Topics related to the Sun Temperature, blackbody radiation, Wien's law The source of the Sun's energy estimating the main sequence lifetime from L and mc2 Fusion and fission The origin of the elements Chapter Three: Topics related to the Moon The history of the Moon's surface The temperature of the Moon's surface radiative equilibrium temperature How rocks form phase diagrams, ternary diagrams, eutectic melt Finding the age of a rock Where did we use: conservation of energy (or mass-energy)? geometry? proportional reasoning?