- 31 - Phy 122 Section 9: Vibration & Waves
advertisement
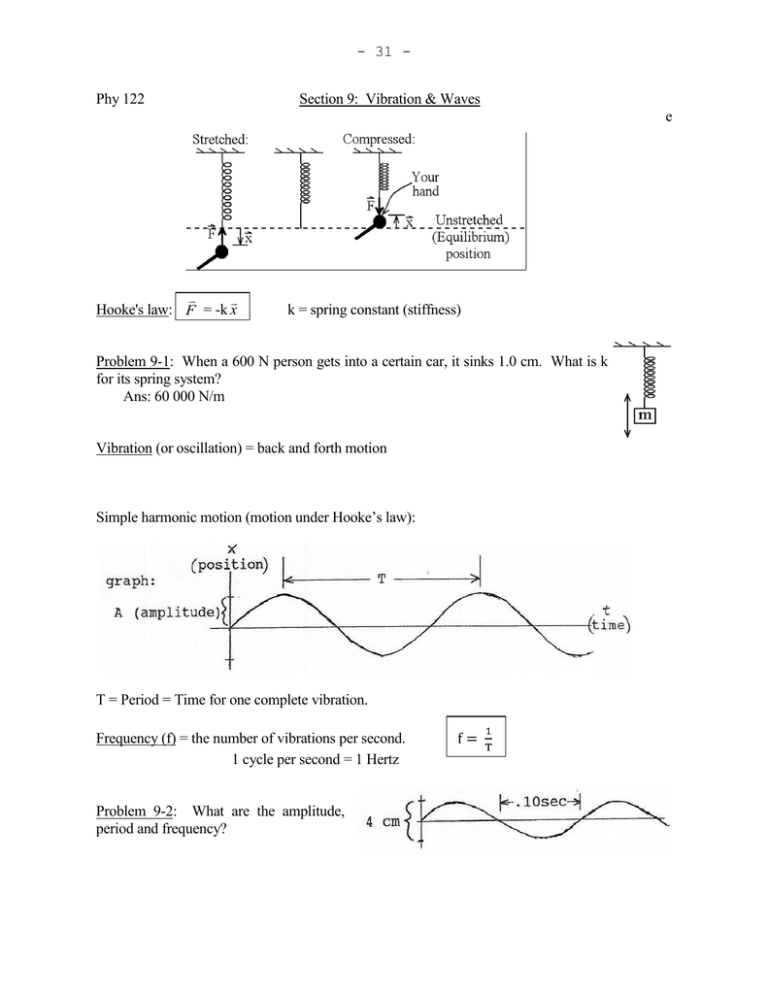
- 31 Phy 122 Section 9: Vibration & Waves e Hooke's law: F = -k x k = spring constant (stiffness) Problem 9-1: When a 600 N person gets into a certain car, it sinks 1.0 cm. What is k for its spring system? Ans: 60 000 N/m Vibration (or oscillation) = back and forth motion Simple harmonic motion (motion under Hooke’s law): T = Period = Time for one complete vibration. Frequency (f) = the number of vibrations per second. 1 cycle per second = 1 Hertz Problem 9-2: What are the amplitude, period and frequency? - 32 - Frequency of a harmonic oscillator: k m where ω = 2πf ω is the frequency in radians per second, f is the frequency in cycles per second. ω is called the “angular frequency,” f is just the “frequency”. Waves: A wave = a moving disturbance. (Sound, water waves, etc. make some material substance move as they go by. Electromagnetic waves, such as light or radio waves, make electric and magnetic fields change strength.) Transverse waves: The medium is displaced perpendicular to the direction of propagation. Examples: light, string waves, water waves (sort of). Longitudinal waves: The medium is displaced parallel to the direction of propagation. Examples: compression waves like sound. Amplitude, A, corresponds to loudness of sound & brightness of light. Frequency, f, corresponds to pitch of sound & color of light. v = Speed: How fast a given crest moves through medium. Speed = distance ÷ time, and a distance of λ corresponds to a time of T, so v = λ ÷ T. Substituting the fact that f = 1/T into this, v=fλ Example: What's the wavelength of middle C (262 Hz)? Ans: λ = v/f = (343 m/s)/(262 Hz) = 1.31 m ↑ speed of sound, from formula sheet. - 33 Problem 9-3: Snapshot of clothesline: You shake one end 3 times per second. Find the wave's frequency, period, wavelength, amplitude & speed. Ans: speed = 3.60 m/s Problem 9-4: The graph represents a sound wave. What is the sound's (a) period? (b) frequency? (c) wavelength? Problem 9-5: WGCC broadcasts at 90.7 megahertz. wavelength. Ans: 11.0 ns, 3.31 m Find the signal’s (a) period and (b) v (wave's speed): depends on the medium, not the wave. (Different frequencies and amplitudes have about the same speed.) - Speed of water waves: Depends on depth of the water. - String waves: v F F = string tension (force) μ = mass per unit length - Speed of sound in air: T= kelvin temperature (Kelvin = °C + 273) Problem 9-6: Waves on a piano string need to travel at 240 m/s. If it is 80 cm long and has a mass of 2.8 grams, how much tension should it be under? Ans: 202 N - 34 Section 10: INTENSITY (of light or sound): unit: W/m2 I = Power area (Power = energy/time.) For a 3 dimensional wave moving in the open, I1 r22 I2 2 1 r 2 (Because area is proportional to r .) Problem 10-1: 10.0 m from a certain lamp, the intensity is 1000 W/m2. For 2000 W/m2, how far from the lamp should you be? Ans: 7.07 m Sound level in decibels: 10 log I 12 10 W / m 2 or I 10 10 12 W / m2 (Every time you multiply I by 10, add 10 dB. For example, 80 dB is 1000 times as intense as 50 dB.) Example 10-a: A sound level meter reads 74 dB. Find the intensity. Ans: 2.51 x 10-5 W/m2 Problem 10-2: 25 m from a machine in an open field, the sound level is 80 dB. a. Find the intensity there. b. What is the intensity 40 m from the machine? c. What is the sound level 40 m from it? Ans: 1.00 x 10-4 W/m2, 3.91 x 10-5 W/m2, 75.9 dB Superposition Principle: Displacement of medium due to several waves at once is the vector sum of the individual displacements. For example, if at some point one wave by itself would pull the water level up by 2 mm, and the other wave alone would pull it down 3 mm, the water level with both waves present at once is 2 + (-3) = -1; 1mm below equilibrium. (+ for up, - for down) - 35 Standing waves: Identical waves going through each other in opposite directions make a standing wave. (For example, shake one end of a clothesline. One wave comes directly from your hand, the other wave has been reflected from the other end.) At certain points, the two waves always give the string equal and opposite pulls, so they cancel at those points. The string moves like so: Notice: λ = 2(distance from node to node) λ = 2(distance from antinode to antinode) λ = 4(distance from node to antinode) Resonance: When a driving force creates a large amplitude vibration. (Due to driving system at a resonant frequency. Shake something at a frequency it naturally vibrates, and you will always be pushing it in the direction it's already going.) A string must have a node at a fixed end. (An end attached to something can't move.) Since nodes are .5λ apart, the string must hold a whole number of half-wavelengths. The special fs obeying that condition are the resonant fs. Example 10-b: Waves on a clothesline 3.0 m long travel at 12 m/s. Find its three lowest resonant frequencies. Ans: 2 Hz, 4 Hz, 6 Hz Harmonic sequence: When all resonant fs are integer multiples of f1. f1 = fundamental frequency = first harmonic f2 = 2f1 = second harmonic f3 = 3f1 = third harmonic etc. A string on a musical instrument typically vibrates in many of these resonant modes at once. The Pitch corresponds to the fundamental frequency. The sound’s Quality corresponds to the relative prominence of the harmonics. Problem 10-3: A string 70 cm long has a mass of 1.3 grams. It is under 150 N of tension. Find a. the speed of waves on this string. (See sec. 8) b. its fundamental frequency. c. its next three resonant frequencies above that. - 36 Ans: 284 m/s, 203 Hz, 406, 609 & 812 Hz Problem 10-4: A guitar string 56.0 cm long produces an A (440 Hz) without fingering. a. What is the speed of waves on this string? b. How far from the end should you put your finger to get a C (523 Hz)? Ans: 493 m/s, 47.1 cm Wind instruments: Standing sound waves in a pipe. Node at a closed end: Antinode at an open end: Example 10-c: A clarinet is closed at one end (by the player's mouth) and open at the other. Find a. the fundamental frequency of a clarinet 60 cm long. b. the next resonant frequency above that. Ans: 143 Hz, 429 Hz Problem 10-5: (a) A flute behaves like a cylindrical tube, open at both ends. If a flute 70 cm long is vibrating in its fundamental mode, what is the sound’s wavelength? (b) Repeat for a tube closed on one end. Ans: 140 cm, 280 cm - 37 Section 11: Electromagnetic waves, such as light, work by induction: A changing electric field induces a changing magnetic field which induces a changing electric field which... For example, the magnetic field around an alternating current changes in proportion to the current, setting the process off. This is how a radio transmitter works. Electromagnetic Spectrum: low f (or long λ): high f (short λ): Radio waves Microwaves Infrared Visible Ultraviolet X - rays gamma rays Roy G. Biv (white = mixture of all λs) These all are basically light with a different frequency. All travel at the same speed in a vacuum: 8 c = 3 x 10 m/s. REFRACTION def.: Index of Refraction: n c v 8 c = speed of light in a vacuum = 3 x 10 m/s v = speed in the material Problem 11-1: How fast does light travel through crown glass? Ans: 1.97 x 108 m/s This bending of light as it crosses from one material into another is called refraction. - 38 - Snell's Law: n1 sin θ1 = n2 sin θ2 Problem 11-2: Find θg: Ans: 29.8° Reflection: Law of Reflection: Angle of incidence = Angle of reflection -Total internal reflection: For example, shine light out from underwater. As you increase θ1, θ2 always has to be larger due to air’s smaller n (Snell’s law), so θ2 gets to 90° first. Beyond this "critical angle," there is no refracted beam; everything goes into the reflected one. Put sinθ2 = 1 in Snell’s law: sin θc = n2 n1 θc = critical angle Example: Find the critical angle for diamond in air. Ans: sin θc = n2 = 1.00 n1 2.42 θc = 24.4° Fiber optics: Due to total internal reflection, light does not escape from inside a glass fiber. (I will demonstrate.) Spherical Concave Mirror (Shown in cross section.) C is the center of curvature. Every point on mirror is same distance R (radius) from point C. F is the focal point; where parallel light rays converge. - 39 - (Thin) Converging Lenses: Ray diagrams: 3 rays easiest to draw accurately: 1. In parallel to axis, out through F. 2. In through F, out parallel to axis. 3. Ray through C reflects back on itself. REAL IMAGE - Rays do pass through image. (So, it can be projected on a screen.) Example: The diagram above. VIRTUAL (or imaginary) IMAGE - Rays don't pass through image. (Can't be projected on a screen.) Example below: (Thin lens similar - see formula sheet.) To solve a problem using a ray diagram: 1. Draw a line across the page to be the axis. 2. Measuring to some convenient scale, a. draw the mirror, its focal point, its center of curvature, and the object, or, b. draw the lens, its two focal points, and the object. - 40 Draw the object with its tail on the axis. Just draw a straight line for the mirror or lens; we are assuming its surface(s) to be small, nearly flat, part(s) of a sphere. or 3. Draw rays from the tip of the object, according to the rules above. They should look something like the diagram on your formula sheet. Any two of the three rays is enough. 4. Look for where the rays cross, which is the tip of the image. (Since the rays come from the same point on the object, they pass through the same point on the image.) Measure the image's size and distance from the lens or mirror. Problem 11-3: An object 10 cm tall is placed 40 cm from a mirror with a radius of curvature of 140 cm. Use a ray diagram to find the image’s A) position, B) size, and C) character. Ans: 93 cm behind mirror, 23 cm, virtual & erect (“Character” means whether it’s real or virtual, and whether it’s inverted or erect.) Hints: (1) With these particular numbers, 1/10 scale works out well if you draw the axis the long way across the page. (2) Place the mirror so there is as much room as possible behind it. To solve a problem like that mathematically: (Magnification: ) do = object distance = dist. from lens (or mirror) to object. di = image distance = dist. from lens (or mirror) to image. (di negative if virtual image.) ho = object height. hi = image height. (positive if erect, negative if inverted.) Example 11-a: Calculate the answer to the previous problem. (10 cm object 40 cm from mirror with R = 140 cm) - 41 Section 12: Interference Or, if you move the top speaker another wavelength, it's the same picture with one more wave drawn onto its left edge. In general, Constructive Interference if path difference = mλ where m = 0 or 1 or 2 or... On the other hand, The positive parts of one wave arrive at the same time as the negative parts of the other, so they cancel. The same thing happens if the path difference is 1½λ or 2½λ or... Destructive Interference if path difference = (m + ½)λ Diffraction = the bending of waves around an obstacle. (For example, you can still hear me with my back turned because the sound diffracts around me.) Diffraction can be used to create two interfering light waves. (Double slit interference) - 42 - (This is the experiment that first proved light is a wave.) With the screen far from the slits, some trig shows that the path difference equals d sin θ. Constructive interference: d sin θ = mλ Destructive interference: d sin θ = (m + ½)λ m = 0, 1, 2, … Problem 12-1: Monochromatic (only one λ) 3800 apart. Find θ for the first order maximum. Ans: .435° light passes through two slits 50 microns Problem 12-2: This pattern is projected on to a wall 2.50 m from the slits. th st How far apart are the 0 and 1 order maxima? (Hint: Consider the triangle shown.) Ans: .0190 m Diffraction grating: Many equally spaced parallel slits (or scratches). Constructive interference at the same angles as with two slits. But, dark if θ is even a little off. d sin θ = mλ Maxima from a grating - 43 - With white light, constructive interference of each λ is at a slightly different angle, separating the light into a spectrum. Problem 12-3: Light from a mercury vapor lamp falls on a grating with 8000 lines per cm. Find nd the angle for the 546 nm line in the 2 order spectrum. Does a third order exist? Ans: 60.9°, no Thin Film Interference: Example: Coating on nonreflective lenses makes all the light go through it, instead of some being reflected: 2t ≈ path difference. Reflected rays cancel each other. If n of film is between ns on either side: Constructive interference: 2nt = mλv Destructive interference: 2nt = (m+ ½)λv m = 0, 1, 2 … λv = wavelength in a vacuum If n of film is higher or lower than n on both sides: Constructive interference: 2nt = (m + ½)λv - 44 Destructive interference: 2nt = mλv The formulas contain n (film’s index of refraction) because slowing the light shortens λ. How the wavelength in the material (λn = λv/n) compares to the path difference (2t) determines what kind of interference there is. The reason for two sets of formulas is that there can be a phase change due to reflection: If both rays or neither are phase shifted, the top box applies. (Notice constructive interference when t ≈ 0.) With one shifted, the bottom applies. (Destructive if t ≈ 0.) With white light, some λs match the thickness of the film to interfere constructively, while others match it destructively. This is why you see colors in bubbles, or a layer of oil floating on water: As the thickness of the film varies between places, the reflected λ varies with it. Problem 12-4: How thick should the coating be to make the lens completely nonreflecting to 550 nm light? Ans: 99.6 nm air n = 1.38 n = 1.55 . glass Single slit interference. Central maximum: θ = 0 Minima: a sin θ = mλ Increasingly faint max.: a sin θ = (m + ½)λ m = 1, 2, 3, … (no 0) Spreading and Resolution: Passing through the opening into an optical instrument spreads light out due to single slit interference: - 45 - a sin θ = (1)λ The image of a pointlike source, like a star, is a blob this big (with fainter light around it) instead of a pointlike image. Two images are thought of as barely resolved if the blobs overlap halfway: Rectangular opening: θmin = λ a Circular opening: θmin = 1.22 λ D D = diameter Problem 12-5: How far from your eye to a pair of barely resolvable headlights 5 feet apart? Assume: 1. Circular pupils 2 mm wide, 2. λ = 500 nm, 3. Only diffraction limits resolution. Ans: 1.64 x 104 ft - 46 Review of Sections 9-12: 1. A certain string on a cello is 88 cm long, and produces an "A" (220 Hz) when bowed or plucked. a. What is the speed of waves on this string? b. If the effective length is reduced to 79 cm by fingering, what is the frequency then? Ans: 387 m/s, 245 Hz 2. An object 2.0 cm tall is placed 4.5 cm from a converging lens with a focal length of 10.0 cm. By constructing a ray diagram, find the image’s position, size and character. Ans: -8.2 cm, 3.6 cm, virtual & erect 14 3. A ruby laser beam (f = 4.32 x 10 Hz) is sent out from a 2.7 m diameter telescope to the moon, 384 000 km away. What is the radius of the red spot it makes on the moon? Ans: 120 m 4. A certain sound has an amplitude of .00001 cm and a frequency of 171.5 Hz. Sketch a graph showing the displacement of the air as a function of position for this wave. Label both axes with appropriate units. 5. Short answer, 5 points each: a. When a diffraction grating is illuminated with white light, which color in the first order spectrum appears closest to the central maximum? b. The speed of light in glass is __________ (greater than? less than? equal to?) the speed of light in diamond. c. The light used in a double slit experiment has a wavelength of .50 μm. Is interference constructive or destructive at a point which is 20.0 μm from one slit and 20.5 μm from the other? d. If you triple your distance from a light source, what happens to the intensity of the light? e. What property of a light wave is perceived as its i. color? ii. brightness? - 47 Section 13: Atoms Wave-particle duality: Experiments in the early 1900’s showed that light is, in some ways, more like a stream of particles than a wave. A good picture (although not perfect) might be to think of light as a lot of little flashes rather than a continuous wave like you see on a pond. Each particle, or flash of light, is called a photon, and has an energy of E = hf -34 h = Planck’s constant = 6.63 x 10 J·s Problem 13-1: What is the energy of a photon with a 500 nm wavelength? Ans: 3.98 x 10-19 J de Broglie wavelength: What’s so special about photons? Maybe all particles have this dual nature, with a wavelength of h mv This was confirmed when electrons, neutrons, and even whole atoms were diffracted from crystals, just like x-rays. Problem 13-2: Calculate the wavelength of a 75 kg person running at 5.0 m/s. Ans: 1.77 x 10-36 m (Because λ is extremely small, the amount you diffract when running through a doorway is too small to measure. So, you don’t notice the wave nature of objects in everyday life.) Demonstration: How can something be both a wave and a particle, when they are as different as black and white? A blob of correction fluid on clear plastic looks white when front lit and black when back lit. It's black or white, depending on how you look at it. Similarly, light is a wave or a particle, depending on whether you look at it in a diffraction experiment, or a photoelectric experiment. (Either model is oversimplified.) The Bohr model of hydrogen (as explained by de Broglie): Think of an electron as a wave on a circular path around the nucleus. To get constructive interference, the circumference of the orbit must be a whole number of wavelengths. 2πr = nπ n = 1 or 2 or 3 or … So, the electron can only exist at certain distances from the nucleus. From Newtonian mechanics and Coulomb’s law, the energies corresponding to these allowed orbits are: - 48 En = (13.6 eV) 2 n -19 1 eV = 1 electron volt = 1.60 x 10 J (energy an electron gains going through one volt.) (The lowest energy level is called the “ground state.” Higher ones are called “excited states.”) Example 13-a: When hydrogen’s electron is in the n = 2 level, the radius of its orbit is 2.12 -10 = 1 angstrom = 10 m.) (a) What is the wavelength of the electron? (b) What is the electron’s energy? (c) How much energy is lost when it drops to n = 1? (d) What wavelength of light is given off dropping to n=1? Ans: 6.66 , -3.40 eV, 10.2 eV, 122 nm Problem 13-3: When the electron in hydrogen is in the n = 3 level, the radius of its orbit is 4.76 (a)What is the wavelength of the electron? (b)What wavelength of light is given off dropping to n=2? Ans: 9.97 , 659 nm . (1 . Spectra: Look at any light source through a grating; you’ll see one of three things: Continuous spectrum: All λs present. Emission spectrum: Only certain λs present. (A pattern of bright colored lines on a dark background.) Absorption spectrum: Only certain λs absent. (Dark lines.) (Pass a continuous spectrum through a gas, and it will absorb the same λs it would emit if hot.) Pattern of lines acts as a "fingerprint" to identify what made the spectrum. For example, this is how we know what stars are made of. No one could explain line spectra, until Niels Bohr suggested that the electrons in atoms are restricted to energy levels. A photon is absorbed or emitted “all or nothing” when an electron jumps or drops from one energy level to another. From what we just went over, he derived: 1 R 1 1 2 f ni n 2 7 -1 R = “Rydberg constant” = 1.097 x 10 m This exactly matches hydrogen’s observed spectrum. Problem 13-4: Calculate the wavelength given off when hydrogen goes from n = 3 to n = 2, the short way. Ans: 656 nm (A little different due to how numbers were rounded.) - 49 Laser: Stimulated emission: spontaneously. Electron is knocked down to a lower energy, instead of falling Example, Helium - neon laser: Neon atoms in tube undergo stimulated emission: Beyond the Bohr Model: A more detailed analysis shows the main energy levels contain sublevels. A complete description needs 4 quantum numbers, not just n: n, l, ml and ms In the n=1 level, there are 2 possible combinations of these numbers. In n=2 there are 8. … (Take chemistry for details.) Pauli Exclusion Principle: No two electrons can have all four quantum numbers the same. So, in multi-electron atoms, the electrons form “shells” because there isn’t room for them all in the lowest level. (2 in the inner shell, 8 in the next, etc.) Solids: (Many atoms forming a crystal lattice; a regular array) Energy bands: When close, atoms disturb each other's energy levels. The atoms’ slightly different levels, taken together, form bands. (Each band is actually many very close levels.) example, sodium at 0 K: Electrons in the band which is partly filled are the ones which flow when there is an electric current. - 50 - Insulators have no partially filled band: Semiconductors (ex: silicon) Doping (adding impurity atoms): Donor: An atom with too many electrons to fit into the lattice gives one off, adding to conduction electrons. n-type semiconductor: Current is mostly electrons. Acceptor: an atom that gains an electron, making a hole. p-type semiconductor: Current is mostly holes. p-n junction: Free charges leave junction. Very small I because few free charges are left to flow. Lots of charges flow in, so I is big. The junction is a diode, conducting in only one direction. (It rectifies: converts AC into pulses of DC.) - 51 - Some holes get stuck on donor atoms in the thin center layer. They repel additional current. The more ib drains off, the larger ie and ic. So small base current controls large e to c current. Used as amplifiers, and as "switches" for digital circuitry. 13-5: a. The energy bands of a certain solid are as shown. Is this material an insulator, a semiconductor or a conductor? b. Does having electrons flow into the middle layer of this transistor increase, decrease, or have no effect on the main current flowing horizontally? c. i. Draw a few holes and electrons in each picture. Draw arrows showing which way they are moving. ii. In which case is the current large? - 52 Section 14: The Nucleus: A nucleus is held together against the repulsion between protons by the Strong force: Protons and neutrons all attract each other. (Very short range: Does not operate outside the nucleus.) Number of protons in nucleus determines which element it is. Isotopes: The different nuclei of an element: Differ in number of neutrons. Example: the 3 isotopes of hydrogen: mass number = total number of nucleons (ps & ns) atomic number = number of protons Example: how many neutrons in a Ans: 208 - 82 = 126 208 82Pb nucleus? 2 Binding energy = (c )(difference between mass of nucleus and particles making it up) Z = atomic number N = no of neutrons Eb (in MeV) = (ZmH + Nmn - matom)(931.5) 12 (masses in atomic mass units: 1 u = 1/12 mass of a C atom) mH = mass of 11H = 1.007825 u mn = mass of neutron = 1.008665 u Problem 14-1: Find the average binding energy per nucleon of 15.994915 u. Ans: 7.98 MeV 16 8O Radioactivity: -Alpha "rays” (least penetrating): example: 238 92U 234 4 90Th + 2He alpha particle (helium nucleus, 2p's & 2n's) Mass numbers add up to same thing on both sides. Atomic numbers add up to same thing on both sides. if the mass of one atom is - 53 -Beta "rays” (intermediate penetration): example: 14 6C 14 0 7N + -1e beta particle (electron) + antineutrino ( : Greek "nu") Neutrino: Does not feel strong force, electromagnetism (no charge), or gravity (little or no rest mass). Therefore, it interacts very weakly with matter. -Gamma rays (most penetrating) are photons. Emitted when an excited nucleus drops to a lower energy level. example: 123 53I 123 53I + γ gamma ray (photon) Problem 14-2: Complete the equations: Half-life: Time for half the nuclei of a substance to decay. example: 14C has a half-life of 5700 years, meaning that an object containing 1.0 gram of 14C 5700 years ago would contain .5 gram today. (The rest decayed into 14N.) 5700 years from now, it would be halved again, to .25 g. N = number of nuclei present R = “decay rate” or "activity" (R = ΔN/Δt) units: SI: 1 becquerel = 1 Bq = 1 decay/sec 10 also popular: 1 curie = 1 Ci = 3.7 x 10 Bq - 54 - Re-arranging that, Problem 14-3: A certain substance has a half-life of five years. If its activity today is 1000 decays/sec, what will its activity ten years from now be? 14 12 Problem 14-4: Radioactive dating: All living things contain the same ratio of C to C, giving 14 each gram of carbon an activity of .25 Bq. The half life of C is 5730 years. If a 100 gram piece of charcoal has an activity of 17 Bq, about how old is it? Ans: 3190 yr Problem 14-5: Iodine 123, half-life 13.2 hours, is used as a medical radioactive tracer. If a dose has an initial activity of 300 μCi, what will the activity be two days later? (In practice you would be exposed to even less, because your body excretes some of it.) Ans: 24.1 μCi Disintegration energy/ reaction energy: Disintegration: Radioactive decay, as above. Reaction: Shooting some particle, a, at a nucleus can transmute it into another element: a+X Y + b + ... 2 Q = (total m before - total m after)c (c2 = 931.5 MeV/u) (Q = KE of Y & other particles, energy of γ rays, etc.) Problem 14-6: Find the Q value for the α decay of 4 222.017574 u, He: 4.002603 u. Ans: 4.87 MeV 226 Ra. Mass of If Q is positive, the process can happen spontaneously. If Q is negative, the process can’t happen spontaneously. Nuclear reactions: Fission: Split a nucleus into smaller ones. examples: original "A" bomb, nuclear reactors. Fusion: Combine nuclei. examples: H-bomb, the Sun. 226 Ra: 226.025406 u, 222 Rn: - 55 Fission: 1 example: 0n + 235 92U 141 56Ba + 92 36Kr + 1 3( 0n) + energy (many other possibilities) (Mass numbers and atomic numbers must add up to same thing on both sides.) 235 140 Problem 14-7: a. Write the equation for 92U splitting into 54Xe and 235 132 101 b. Write the equation for 92U splitting into 50Sn and 42Mo. Chain reaction: The neutrons released go on to split other etc. 94 38Sr. 235 U's, releasing even more neutrons, Fusion: Example: Requires great temperature and/or pressure. Effects of radiation: Ionizing radiation damages molecules in cells. If the cell can't repair the damage: - Radiation sickness: Many dead/damaged cells, causing blood abnormalities at a certain dosage; nausea, hair loss, etc at a higher dosage; death at a still higher dose. - Cancer: If there is damage to a cell's genetic material, it can start dividing out of control. -Birth defects: Damage to genes in reproductive cells can cause mutations, passed down to all future generations. - 56 Review of Sec. 1 - 14: 1. How many amps are consumed by the 5.00 Ω resistor? Ans: 1.50 A 2. Assume that a clarinet is a cylindrical tube full of air at room temperature (speed of sound = 343 m/s), 60 cm long, open at one end and closed at the other. a. Find the frequency and wavelength of the lowest note which can be played on it with all the side holes shut. b. Find the frequency and wavelength of the next lowest note. (It’s not just twice the frequency from a.) Ans: 143 Hz, 2.40 m, 429 Hz, .800m 3. Light falling on a pair of glass plates reflects from the top and bottom of the space between them. At one particular point, there is a dark interference fringe when 420 nm light is used. At this same point, there is a bright interference fringe when 504 nm light is used. What is the narrowest possible value for the gap between the plates at this point? Ans: 630 nm 4. Consider the nuclear reaction 2H + 6Li → 24He. The masses of these nuclei are: 2H, 2.014 102 u; 6Li, 6.015 122 u; and 4He, 4.002 603 u. Assume the hydrogen and lithium nuclei collide at very low speeds, meaning that their initial energies are entirely in the form of mass. What is the total kinetic energy, in joules, of the helium nuclei after the reaction? Ans: 3.58 x 10-12 J -18 5. In an electrostatic air cleaner, a piece of dust with a charge of 3.68 x 10 C is between charged parallel plates 8.00 mm apart. If there are 120 V between these plates, how large is the force on the piece of dust, in newtons? Ans: 5.52 x 10-14 N 6. One coil is placed around another as shown. The solenoid has a cross sectional area of.028 m2 and has 700 turns per meter of length. The second coil has 130 turns. The current in the solenoid goes from 0 to 10 A in .002 s. Find the voltage induced in the second coil during this time. Ans: 16.0 V 7. To determine the inductance of a coil, you put it in a circuit as shown, and notice that the AC ammeter reads 1.60 A. What is L? - 57 Ans: 63.7 nH 8. Short answer, 5 points each: a. Assume the inductor has no resistance. Which point (A or B) will be at the higher potential when the current is i. increasing? ii. decreasing? b. The bubble is 1/4 of a wavelength thick (using the wavelength in the liquid). Will the reflected rays interfere constructively, destructively, or do something in between? c. A hydrogen atom in its ground state (n = 1) absorbs a photon. What is the smallest possible energy this photon could have? (Refer to the energy level diagram for hydrogen at right.) d. Two long ropes, a fat one and a thin one, are under the same tension. Waves with the same period are traveling along both. Do these waves also have the same wavelength? e. What is the more common name for a coulomb per second?







