Take Home Exam 1 - Chem 434 -Spring 2003
advertisement
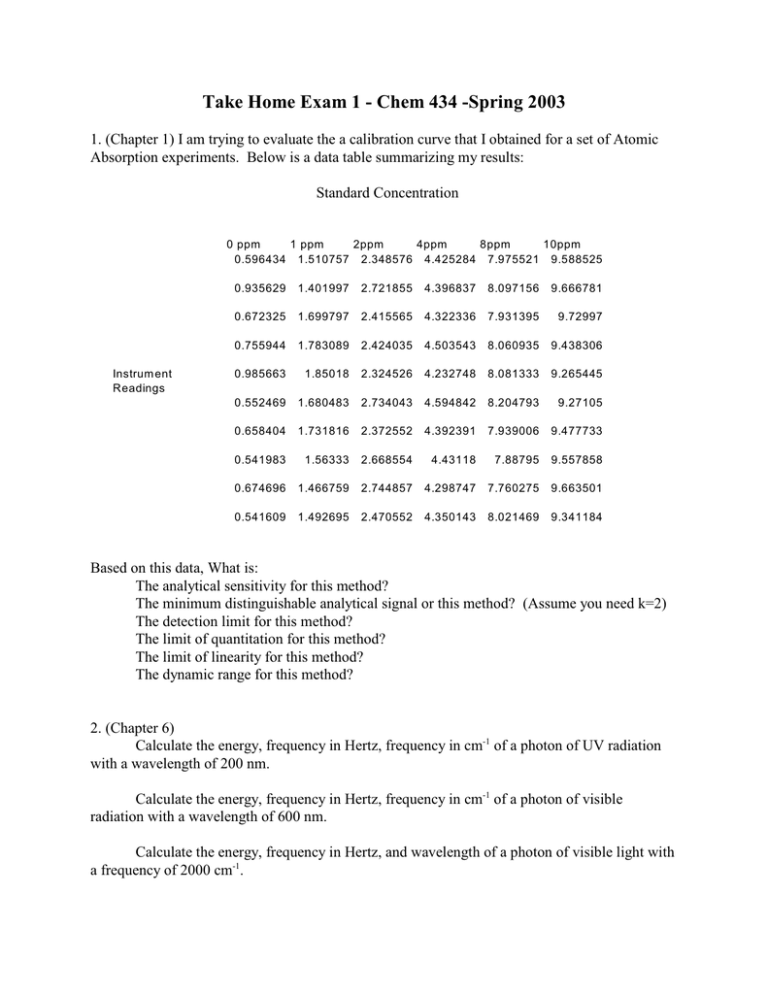
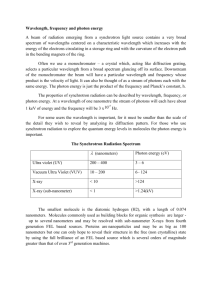
Take Home Exam 1 - Chem 434 -Spring 2003 1. (Chapter 1) I am trying to evaluate the a calibration curve that I obtained for a set of Atomic Absorption experiments. Below is a data table summarizing my results: Standard Concentration 0 ppm 1 ppm 2ppm 4ppm 8ppm 10ppm 0.596434 1.510757 2.348576 4.425284 7.975521 9.588525 Instrum ent Readings 0.935629 1.401997 2.721855 4.396837 8.097156 9.666781 0.672325 1.699797 2.415565 4.322336 7.931395 9.72997 0.755944 1.783089 2.424035 4.503543 8.060935 9.438306 0.985663 1.85018 2.324526 4.232748 8.081333 9.265445 0.552469 1.680483 2.734043 4.594842 8.204793 9.27105 0.658404 1.731816 2.372552 4.392391 7.939006 9.477733 0.541983 1.56333 2.668554 4.43118 7.88795 9.557858 0.674696 1.466759 2.744857 4.298747 7.760275 9.663501 0.541609 1.492695 2.470552 4.350143 8.021469 9.341184 Based on this data, What is: The analytical sensitivity for this method? The minimum distinguishable analytical signal or this method? (Assume you need k=2) The detection limit for this method? The limit of quantitation for this method? The limit of linearity for this method? The dynamic range for this method? 2. (Chapter 6) Calculate the energy, frequency in Hertz, frequency in cm-1 of a photon of UV radiation with a wavelength of 200 nm. Calculate the energy, frequency in Hertz, frequency in cm-1 of a photon of visible radiation with a wavelength of 600 nm. Calculate the energy, frequency in Hertz, and wavelength of a photon of visible light with a frequency of 2000 cm-1. 3. (Chapter 7) In some Instrumentation classes, you actually have to put an instrument together. Let’s assume that your assignment is to build a single beam visible spectrometer, and a single beam IR spectrometer. For each of the components below, compare and contrast the components you would use to build these two machines. Why did you chose this particular component for your instrument. Source Wavelength selector Windows, lenses or prisms Detector Sample holder 4. (Chapter 8) In problem 2 you calculated the energy of a photon of UV, visible, and IR light. This also corresponds to the energy needed to move a molecule from a ground state to an excited state using this radiation. In the next chapter we will start with the assumption that a molecule is always in its ground state, and that no molecules are in the excited state. I want to see if this is true. Use the Bolzmann equation (8-1) to determine how may molecules are already in an excited state due to the temperature - at room temp (25oC ), in a Bunsen burner flame (1800oC) and in an ICP flame (6000K), for each of these transitions. 5. (Chapter 9) Let’s say you just moved in to an old house, and you want to know if the wall have lead paint on them. You have chipped off about a 1 g sample of paint from a wall for analysis. Describe, in as much detail as possible how you will go to the lab and analyze this sample for lead. To help you get started I have included copies of the pages out of the Atomic Absorption manual that describe the parameters for Pb analysis. Things I want to see in your analysis: Which do you expect to be more sensitive Absorption or Emission? What wavelengths and slit widths will you use and why? What sensitivity do you expect to see at these settings? What will you use as your standard(s)? Are there any precautions you want to use? How do you think you will prepare your sample? This is a wide open question. Feel free to use the Web or library resources to see if you can find a procedure you can use directly, or you think you might be able to modify, just make sure you cite a reference for the procedure. If you are interested, I could turn this into a more of a term paper type assignment, rather than a 1 day test question.