Name:_____________ Chemistry 114 Fourth Hour Exam
advertisement
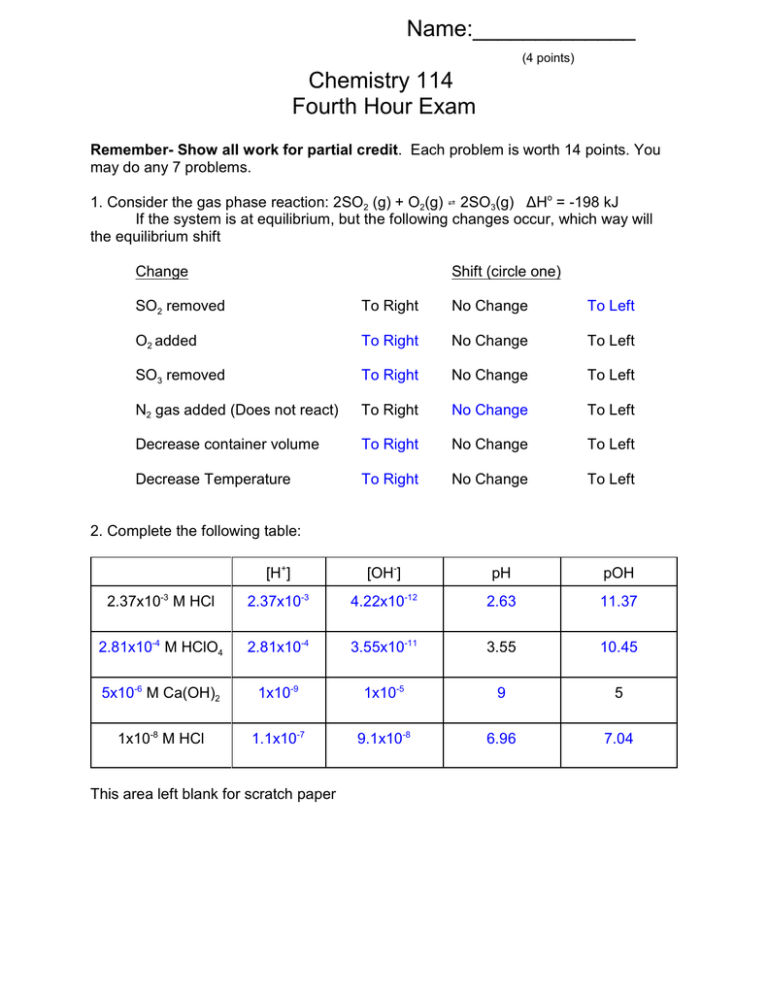
Name:_____________ (4 points) Chemistry 114 Fourth Hour Exam Remember- Show all work for partial credit. Each problem is worth 14 points. You may do any 7 problems. 1. Consider the gas phase reaction: 2SO2 (g) + O2(g) W 2SO3(g) ÄHo = -198 kJ If the system is at equilibrium, but the following changes occur, which way will the equilibrium shift Change Shift (circle one) SO2 removed To Right No Change To Left O2 added To Right No Change To Left SO3 removed To Right No Change To Left N2 gas added (Does not react) To Right No Change To Left Decrease container volume To Right No Change To Left Decrease Temperature To Right No Change To Left 2. Complete the following table: [H+] [OH-] pH pOH 2.37x10-3 M HCl 2.37x10-3 4.22x10-12 2.63 11.37 2.81x10-4 M HClO4 2.81x10-4 3.55x10-11 3.55 10.45 5x10-6 M Ca(OH)2 1x10-9 1x10-5 9 5 1x10-8 M HCl 1.1x10-7 9.1x10-8 6.96 7.04 This area left blank for scratch paper 3. I am going to put 0.150 mole of an acid into 1 liter of water. If this acid becomes 7% ionized... A. What is the concentration of the acid in the un-ionized form? 7% ionized means 93% un-ionized 93% = un-ionized/total x 100% 93/100 = X/.150 .93 x .150 - un-ionized = .1395M B. What is the concentration of the acid in the ionized form? 7% = ionized/total x 100% 7/100 = X/.150 .07 x .150 - un-ionized = .0105M C. What is the pH of the solution? [H+] = [A-] = ionized = .0105 pH = -log (.0105) = 1.98 D. What is the Ka of the acid? Ka=[H+][A-]/[HA] = (.0105)(.0105)/.1395 = 7.9x10-4 4. Rate the following compounds as Strong Acids (SA), Weak Acids (WA), Neutral(N), Weak Base (WB) OR Strong base (SB) H2SO3 ___WA_______ NH3 ___WB_______ K2 O ___SB_______ H2SO4 ___SA_______ CO2 ___WA_______ NaNO3 ____N______ CH3NH2 ___WB_______ 5. Define or give an equation for the following terms: pOH -log[OH-] 2nd Law of thermodynamics In any spontaneous process the entropy of the universe increases Enthalpy H=E+PV = sum of kinetic and potential E of all particles in a system Path function A function that depends on the path taken between two states Intensive property A property of a system that does not depend on the amount of material in the system Buffer A solution that resists changes to pH whenever acid or base is added to the solution 6A . In the following process does the ÄS of the system: Increase (8) Decrease (9) remain the same (NC) or cannot be determined (??) A car is built ____9______ A car is smashed in a wreck ____8______ NaCl(s) WNa+(aq) + Cl-(aq) _____8_____ Ice Freezing at -50 C ____9______ H2O(l)6H2O(g) at 400K ____8______ H2O(l)6H2O(g) at 300K ____8______ A mixture of oil and water separates into two phases ____9______ 6B. In the following process does the ÄS of the universe: Increase (8) Decrease (9) remain the same (NC) or cannot be determined (??) A car is smashed in a wreck ____8______ Ice melting at -5o C ____9______ Ice Freezing at +50 C ____9______ NaCl(s) 6Na+ (aq) + Cl-(aq) ____8______ H2O(l)6H2O(g) at 400K ____8______ H2O(l)6H2O(g) at 300K ____9______ A mixture of oil and water separates into two phases ____8______ 7. At what temperatures will the following process be spontaneous A. ÄH = +19 kJ ÄS = -60 J/K Never Spontaneous B. ÄH = +19 kJ ÄS = +60 J/K 19000/60 = 316.7K Spontaneous at T> 316.7 C. ÄH = -19 kJ ÄS = -60 J/K 19000/60 = 316.7K Spontaneous at T< 316.7 D. ÄH = -19 kJ ÄS = +60 J/K Always spontaneous 8. The overall reaction for the corrosion of iron by oxygen (rusting) is 4Fe(s) + 3O2(g) W 2Fe2O3(s) Using the following data, calculate the equilibrium constant for this reaction at 25oC Substance Fe2O3(s) Fe(s) O2(g) ÄHf o(kJ/mol) -816 0 0 So(J/K@mol) 90 25 225 ÄH = 2(-816) - [4(0) + 3(0)] = -1632 kJ ÄS = 2(90) - [4(25)+3(225)] = 180-(100+675) = -595 J ÄG = ÄH -TÄS = -1632000J - 298(-595) =-1632000 +177310 J =-1455000 J ÄG= -RTln(K) -1455000 = -8.3145(298) ln(K) -1455000/(-8.3145 x 298) = ln(K) 587 = ln(K) e587 = K TI-84's will give an overflow error a this point but some calculators will say K = 9.5x10254