Chemistry 112 First Hour Exam
advertisement
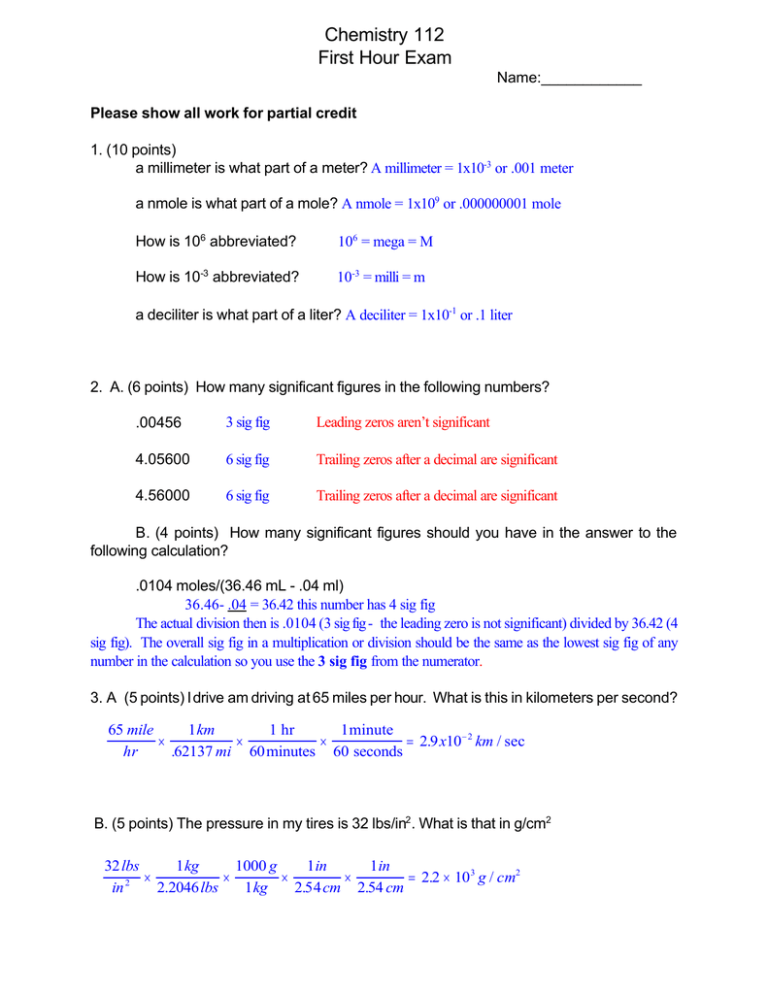
Chemistry 112 First Hour Exam Name:____________ Please show all work for partial credit 1. (10 points) a millimeter is what part of a meter? A millimeter = 1x10-3 or .001 meter a nmole is what part of a mole? A nmole = 1x109 or .000000001 mole How is 106 abbreviated? 106 = mega = M How is 10-3 abbreviated? 10-3 = milli = m a deciliter is what part of a liter? A deciliter = 1x10-1 or .1 liter 2. A. (6 points) How many significant figures in the following numbers? .00456 3 sig fig Leading zeros aren’t significant 4.05600 6 sig fig Trailing zeros after a decimal are significant 4.56000 6 sig fig Trailing zeros after a decimal are significant B. (4 points) How many significant figures should you have in the answer to the following calculation? .0104 moles/(36.46 mL - .04 ml) 36.46- .04 = 36.42 this number has 4 sig fig The actual division then is .0104 (3 sig fig - the leading zero is not significant) divided by 36.42 (4 sig fig). The overall sig fig in a multiplication or division should be the same as the lowest sig fig of any number in the calculation so you use the 3 sig fig from the numerator. 3. A (5 points) I drive am driving at 65 miles per hour. What is this in kilometers per second? 65 mile 1 km 1 hr 1 minute × × × = 2.9 x10 − 2 km / sec hr .62137 mi 60 minutes 60 seconds B. (5 points) The pressure in my tires is 32 lbs/in2. What is that in g/cm2 32 lbs 1 kg 1000 g 1 in 1 in × × × × = 2.2 × 10 3 g / cm2 2 in 2.2046 lbs 1 kg 2.54 cm 2.54 cm 2 4. (10 points) What is the difference between an ionic bond and a covalent bond. What kinds of atoms are involved in each bond? Covalent bond - electrons shared between the two atoms- atoms are held together by the electrons shared between them - both atoms are nonmetals Ionic bond - electrons are not shared equally between the atoms. One atom, the anion, has one or more electrons more than its atomic number, giving it a negative charge, while a second atom, the cation, has one or more electrons less than its atomic number, giving it a positive charge. The atoms are held together by the electrostatic attraction between the negatively charged anion and the positively charged cation. The cation is usually a metal while the anion is a nonmetal. 5. (5 points) What is the chemical name of the element with an atomic mass of 41 and an atomic number of 20. Do you think this is the most common isotope of this element? 41 20 Ca If you look on the periodic table you see that the average atomic weight of Ca is 40.08 which is 40 almost an entire atomic mass unit less than this isotope. Thus the most common isotope is probably 20 Ca 41 and the 20 Ca isotope is probably not common. 6. (10 points) Give the name (NOT abbreviations) of: Any alkali metal Lithium, Sodium, Potassium, Rubidium, Cesium, or Francium An inert gas in the third period Argon An alkaline earth metal in the second period Beryllium Any transition metal Any metal between Scandium, Zinc, Lanthanum, and Mercury Fluorine is a member of which chemical family halide or halogen 7. Name the following compounds (3 points each) SF6 Sulfur hexafluoride FeCl2 Iron(II) chloride K2SO4 Potassium sulfate Give the molecular formula of the following compounds (3 points each) Dinitrogen tetraoxide N2O4 Calcium chloride CaCl2 3 8. (10 points) What is the % composition of each of the elements in Sr(OH)2 Grams of each element in 1 mole of compound Sr = 87.62 x 1 = O = 16.00 x 2 = H = 1.008 x 2 = total of atomic masses = 87.62 32.00 2.016 121.636 divide mass of individual elements by total mass % Sr = 87.62/121.636 = % O = 32.00/121.636 = % H = 2.016/121.636 = 72.03% 26.31% 1.657% 9. (10 points) A compound has a molecular weight of 46.068 contains 52.17%C, 13.13% H and 34.73% oxygen. What is the molecular formula of this compound? Multiply molecular weight by percent composition to find mass of each element in compound: 46.068 x .5217 = 24.0337 g C 46.068 x .1313 = 6.0487 g H Divide mass of each element in compound by atomic mass of element to find number of moles of element in compound. 24.0337/12.01 = 2.00 = 2 moles 6.0487/1.008 = 6.001 = 6 moles 15.999/16.00 = .99994 = 1 mole C2H6O 10. A (4 points) Balance the following equation: K O H K O H K(s) + H2O(l)6 KOH(aq) + H2(g) K(s) + H2O(l)6 KOH(aq) + H2(g) 1 =1 1 = 1 2 … 1 2 K(s) + H2O(l)6 KOH(aq) + ½ H2(g) 1 =1 1 = 1 2 = 1 1 Balanced Balanced Not balanced unless use coefficient of ½ for H2, then Balanced Balanced Balanced As a general rule you should not have fractional coefficients so multiply all the numbers by 2 to get rid of the ½ 2K(s) + 2H2O(l)6 2KOH(aq) + 1 H2(g) 4 B (6 points) Give a balanced equation for the following reaction: Solid iron (III) sulfide + hydrogen chloride gas reacts to give solid iron(III) chloride and hydrogen sulfide gas. iron (III) sulfide = Fe2S3 hydrogen chloride = HCl iron(III) chloride = FeCl3 hydrogen sulfide = H2S Next put in equation form and add the physical states Fe2S3(s) + HCl(g) 6FeCl3(s) + H2S(g) Now start balancing Fe2S3(s) + HCl(g) 6FeCl3(s) + H2S(g) Fe 2 1 (Need 2 FeCl3 on right to balance) Fe Cl Fe2S3(s) + HCl(g) 62FeCl3(s) + H2S(g) 2 2 1 6 Fe Cl S Fe2S3(s) + 6HCl(g) 62FeCl3(s) + H2S(g) 2 2 6 6 3 1 (Need 3 H2S on right to balance) Fe Cl S H Fe2S3(s) + 6HCl(g) 62FeCl3(s) + 3H2S(g) 2 2 6 6 3 3 6 6 Balances with no changes, hooray! Final balanced equation: Fe2S3(s) + 6HCl(g) 62FeCl3(s) + 3H2S(g) (Need 6 HCL on left to balance)