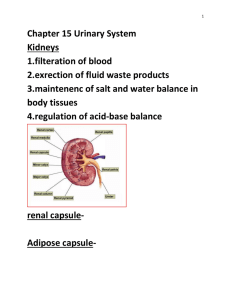
Science in Animal Science presented on November 14. 1990 Concentrations

AN ABSTRACT OF THE THESIS OF
Patrice L. Prater for the degree of Master of
Science in
Animal Science presented on November 14. 1990
Title:
Physiological Factors Affecting Ovine Uterine
Estrogen and Progesterone Receptor
Concentrations
Abstract Approved:
Redacted for Privacy
w-A
Fredrick Stormshak
Two experiments were conducted to determine whether in ewes uterine concentrations
of estrogen and progesterone
receptors are affected by the presence of a conceptus or by the hormonal milieu associated with extremes in photoperiod to which ewes are exposed.
In Exp.1, nine mature ewes were unilaterally ovariectomized by removing an ovary bearing the corpus luteum
(CL).
The ipsilateral uterine horn was ligated at the
external bifurcation and a portion of the anterior ipsilateral uterine horn was removed and assayed for endometrial nuclear and cytosolic concentrations of estrogen receptor (ER) and progesterone receptor (PR) by exchange assays.
After a
recovery estrous cycle, ewes were bred to a fertile ram.
On day 18 of gestation a 10 ml jugular blood sample was collected for measurement of serum concentrations of estradiol -178 (E2) and progesterone by radioimmunoassay.
Ewes were
relaparotomized on day 18 and the remaining uterine tissue was removed.
Endometrium from both the pregnant and nonpregnant uterine horn was assayed for nuclear and cytosolic ER and PR concentrations.
Nuclear and cytosolic ER concentrations on day 10 of the cycle were greater than in endometrium of gravid and nongravid uterine horns on day 18 of gestation (p<.01).
Endometrial nuclear PR levels were also greater on day 10 of the cycle than in the pregnant (p<.05) and nonpregnant horn
(p<.01) on day 18 of gestation.
There were no differences in nuclear and cytosolic ER and PR concentrations between the pregnant and nonpregnant uterine horn on day 18.
Serum levels of E2 and progesterone on day 18 of gestation were 16.56 ±
2.43 pg/ml and 1.74 ± 0.57 ng/ml, respectively.
These data suggest that duration of exposure of the uterus to progesterone and(or) the presence of the conceptus causes a reduction in uterine concentrations of ER and PR.
Further, an effect of the conceptus, if any, is exerted via a systemic route.
In Exp.
2, ten mature ewes were bilaterally ovariectomized in early October.
During the onset of the
winter solstice (late December), a 10 ml blood sample was collected from five ewes for analysis of serum levels of E2 and progesterone.
Ewes were then laparotomized and approximately one-third to one-half of a uterine horn was removed and assayed for endometrial nuclear and cytosolic ER.
The contralateral horn was ligated at the external bifurcation and 10 Ag of E2 in 3 ml of physiological saline was injected
into the uterine lumen of the ligated horn.
After 48 h, a jugular blood sample was collected for steroid analysis and a section of the E2_treated horn was removed and assayed for endometrial cytosolic and nuclear ER.
This procedure was repeated on the remaining five ewes during the height of the summer solstice (late June).
Endometrial nuclear and cytosolic concentrations of ER prior to and after exogenous
E2 stimulation were similar during the winter and summer
solstice (p>.05).
However, treatment with
E2 increased endometrial nuclear and cytosolic concentrations of ER compared with those of the nonstimulated uterine horn during the winter and summer solstice (p<.05 for each).
Serum levels of E2 prior to luminal treatment of ewes with E2 during the winter and summer solstice did not differ (16.55 ± 4.05 vs
16.00 ± 3.0 pg/ml, respectively, p>.05).
Serum levels of E2
48 h after administration of E2 did not differ among ewes at the winter and summer solstice (18.75 ± 2.4 vs 18.65 ± 1.65
pg/ml, respectively, p>.05).
Serum levels of progesterone were basal (<0.10 ng/ml) and did not differ in ewes prior to
and after E2 treatment at the winter and summer
solstice
(p>.05).
These data indicate that physiological factors
and(or) hormones such as prolactin and melatonin secreted in response to extremes in photoperiod do not appear to influence uterine concentrations of ER in ovariectomized ewes.
Physiological Factors Affecting Ovine Uterine
Estrogen and Progesterone Receptor Concentrations by
Patrice L. Prater
A THESIS submitted to
Oregon State University in partial fulfillment of the requirements for the degree of
Master of Science
Completed November 14, 1990
Commencement June, 1991
APPROVED:
Redacted for Privacy
Professor of Animal Science and Biochemistry and Biophysics in charge of the major
Redacted for Privacy
Head of Department of Animal Science
Dean of C
Redacted for Privacy
Date thesis presented:
Typed for Researcher by:
November 14, 1990
Patrice L. Prater
ACKNOWLEDGEMENTS
I would like to take the opportunity to express my thanks
to all of the individuals who helped make this thesis a
reality.
Sincere thanks to Dr. "Stormy" Stormshak for his guidance and friendship, and most importantly, for teaching me perseverance to "take the bull by the tail on a uphill pull" as there will always be worthwhile and attainable goals to pursue.
I would also like to thank Ligaya Tumbelaka, Anna
Cortell-Brown, Ov Slayden, and Sue Leers-Sucheta for their help and friendship during surgeries and laboratory tribulations.
Appreciation also to the U.S.F.S, LaGrande research station and Dr. John Stellflug of the U.S. Sheep
Experimental Station, Dubois, Idaho for their contributions toward this research.
Thanks also to Dr. Kenneth Rowe, Oregon
State University, for his statistical guidance.
In hindsight,
I realize that without the support and
encouragement of those most dear to me, this degree would not have been realized.
Therefore, I would like to extend a very special thank you to my family (Moores and Praters) and my
husband Brian for believing in me and for giving me the
foundation of love and encouragement on which to build.
An extra special thank you to Brian, who as a husband and best friend has shown me that prosperity in life is found through love and laughter.
TABLE OF CONTENTS
Page
REVIEW OF LITERATURE
Introduction
1
1
ESTROGEN AND PROGESTERONE
Origin of Estrogen and Progesterone
Physiological Responses to Estrogens
Physiological Responses to Progesterone
Biosynthesis of Estrogen and Progesterone
Steroid Hormone Transport
Steroid Hormone Receptors
THE ESTROUS CYCLE
Follicular Phase
Luteal Phase
Luteolysis
17
17
18
20
EARLY GESTATION
Maternal Recognition of Pregnancy
Implantation or Attachment
23
23
26
SEASONAL REPRODUCTION
Melatonin
Prolactin
30
31
34
EXPERIMENT 1
Introduction
Materials and Methods
Animals
Nuclear Estrogen Receptor Assay
Cytosolic Estrogen Receptor Assay
Nuclear Progesterone Receptor Assay
Cytosolic Progesterone Receptor Assay
DNA Determination
Serum Progesterone Radioimmunoassay
Serum Estradiol Radioimmunoassay
Statistical Analysis
Results
Discussion
EXPERIMENT 2
55
Introduction
55
Materials and Methods
56
Animals
56
Nuclear and Cytosolic Estrogen Receptor Assay 57
38
38
39
39
40
42
43
44
45
46
47
49
49
52
7
11
12
4
4
5
7
TABLE OF CONTENTS (CONT.)
DNA Determination
Serum Progesterone and Estradiol
Radioimmunoassay
Statistical Analysis
Results
Discussion
BIBLIOGRAPHY
Page
57
58
58
58
61
64
LIST OF FIGURES
Figure
1.
Specific binding of Clflestradio1-178 to nuclear and cytosolic estrogen receptors (mean ±
SE) in endometria of ewes on day 10 of the estrous cycle and in endometria of gravid and non-gravid uterine horns of ewes on day 18 of gestation.
Mean nuclear and cytosolic concentrations of estrogen receptors, day 10 vs day 18 gravid and nongravid horns differ (p<.05).
Page
51
2.
Specifically bound (3H)R5020 to nuclear progesterone receptors (mean ± SE) in endometria of ewes on day 10 of the estrous cycle and in endometria of the gravid and nongravid uterine horns of ewes on day 18 of gestation.
Day 10 vs day 18 gravid and nongravid horns differ in concentrations of progesterone receptors (p<.05).
51
3.
Specifically bound [3H]estradio1-178 to nuclear and cytosolic estrogen receptors (mean ± SE) in nonstimulated and exogenous estradiol-stimulated endometria of ewes during the winter and summer solstice.
Stimulated vs nonstimulated endometrium; nuclear (p<.05), cytosolic (p<.01).
60
PHYSIOLOGICAL FACTORS AFFECTING OVINE UTERINE
ESTROGEN AND PROGESTERONE RECEPTOR CONCENTRATIONS
REVIEW OF LITERATURE
INTRODUCTION
The mechanisms of steroid hormone action have long been an area of fascination and scientific inquiry.
In the female, estrogen and progesterone play a primary role in promoting the
development of an intrauterine environment necessary
for successful procreation.
Prior to 1960 most research on estrogens and progesterone entailed studies with various mammalian species to elucidate the physiological effects of these steroids on the development of the uterus and mammary glands.
During the 1960's interest in the effects of these hormones shifted from physiological to biochemical.
Indeed, it was during the late 1960's that experimental evidence was first presented to suggest that the biological responses evoked by estrogens were the consequence
of this steroid being bound within the target cell by a
macromolecular receptor.
Subsequently, research demonstrated the existence of target tissue receptors for other steroids, including progesterone.
Because the availability of estrogen and progesterone receptors directly affects the biological activity of these steroids,
much research has
been conducted to quantify
2 estrogen and progesterone receptors in uteri of laboratory animals.
However, comparatively little is known about the changes in uterine concentrations of estrogen and progesterone receptors that occur during various reproductive states in domestic animals.
One of the leading causes of reduced reproductive efficiency in many species is embryonic mortality.
Most embryos are lost before or soon after implantation.
Many factors influence the ability of an embryo to implant including adequate production of ovarian hormones and appropriate uterine responses to these hormones.
However, whether embryo wastage is, in part, attributable to impaired
action of estrogen and progesterone
is unknown.
information on estrogen and
Basic progesterone receptor concentrations in the uterus during early gestation could provide insight about factors that maintain or interfere with successful pregnancy.
In the northern hemisphere, sheep are seasonal breeders that exhibit behavioral estrus during the fall and winter and then become anestrus during the late spring and early summer.
The underlying cause for the annual reproductive cycle of ewes
has been attributed to changes in duration of melatonin secretion that occurs in response to seasonal changes in
photoperiod.
Secretion of melatonin from the pineal gland of ewes is maximal as hours of daylight decrease and minimal as hours of daylight increase.
In a number of species, daily
3 profiles of secretion of melatonin and prolactin are inversely related.
Indeed, experimental evidence suggests that melatonin can inhibit the secretion of prolactin from the adenohypophysis.
It is not known whether systemic concentrations of prolactin
or melatonin can
alter the
responsiveness of the uterus of ewes to steroids.
These hormones have been implicated as having a direct effect on the
uterus in the mink, pig, hamster and rabbit.
Therefore, research is needed to determine whether uterine concentrations of steroid receptors in the ewe are influenced by seasonal fluctuations in the secretion of melatonin and prolactin.
The following review of literature will encompass the
physiological and biochemical responses of the uterus to
estrogen and progesterone.
Much of the review will rely on data acquired from the study of laboratory animals but when available, information on domestic animals and primates will be included.
4
ESTROGEN AND PROGESTERONE
ORIGIN OF ESTROGEN AND PROGESTERONE
The female mammal is born with all of the oocytes she will need throughout her reproductive life.
In order for an oocyte to be ovulated it must first go through several stages of maturation.
In the initial stages of folliculogenesis,
each oocyte is surrounded by a single layer of
granulosa cells, together forming a primordial follicle (Ireland, 1987).
Just prior to or following birth the primordial
follicle enters and remains in a resting state until the appropriate hormonal signals cue it to resume maturation in preparation for ovulation or atresia (Ireland, 1987).
Follicular growth
and maturation occur
in a series of events whereby the
primordial follicle first develops into a
primary, then secondary follicle, followed by formation of the fluid filled
tertiary follicle and finally the ovulatory or Graafian
follicle (Bjersing, 1967;
Turnbull et al., 1977).
Only follicles that undergo "recruitment" actually ovulate.
Most follicles instead become atretic and are subsequently replaced
by new follicles
(Smeaton and Robertson, 1971).
In the
bovine, this maturation process occurs in sequential waves of development until the ensuing ovulation occurs (Sirois and
Fortune, 1988).
Soon after ovulation, the ruptured follicle is transformed into a corpus luteum (CL).
5
During the estrous or menstrual cycle, ovarian follicles and the CL are the primary sources of steroid synthesis and secretion (Falck, 1959; Hafs and Armstrong, 1968; Moor, 1977).
Follicle steroidogenesis begins as the specialized cells that comprise the follicle wall (granulosa and theca cells) grow and proliferate.
It has been well established that follicles are the primary source of estrogens in the nonpregnant animal.
Similarly, once the CL has formed it also becomes a major source of steroid synthesis with progesterone being the major hormone secreted. During pregnancy, depending on the species, the developing conceptus and later the placenta may become a
rich source of estrogens and progestins.
Estrogens and progestins are also synthesized and secreted by the adrenal cortex, however,
this source accounts
for
only a minor
fraction of the total estrogen and progesterone
in the systemic circulation. Without steroid synthesis and secretion the complex
and highly
synchronized reproduction could not occur.
events that permit
PHYSIOLOGICAL RESPONSES TO ESTROGENS
Depending upon the mammalian species, there are several forms of estrogens that are synthesized and throughout the body.
However, circulate
the most
important and prevalent of the estrogens are estradio1-17B and estrone.
Estradio1-17B is approximately ten times more potent than estrone and is the most biologically active of all steroids
6 produced by the ovary (Hisaw, 1959).
A third estrogen known as estriol is found in high concentrations in primates during gestation.
In general, estriol is considered the least potent of these three estrogenic forms (Hisaw, 1959).
During the estrous or menstrual cycle, estrogens are responsible for stimulating cellular proliferation and growth of the female reproductive tract, regulation of utero-ovarian function and intrauterine environment, and development and maintenance of secondary sex characteristics.
The following discussion will focus on the role of estrogens in regulating utero-ovarian function and the intrauterine environment.
Estrogens act on the uterine myometrium to increase both the frequency and amplitude of contractions, which are requisite for sperm and accelerated ovum transport, and in some species, migration and spacing of embryos (Pope et al.,
1982). Estrogens initiate endometrial stromal edema (Astwood,
1938) as a result of increased uterine capillary and venule permeability (Halkerston et al., 1960; Martin et al., 1973;
Espey, 1980).
In response to estrogens, the glands of the endometrium become increasingly tortuous as the nuclear RNA polymerases and mRNA activate and facilitate protein synthesis, cell hypertrophy and cell hyperplasia.
Estrogens are also responsible for increasing uterine blood flow
(Williams, 1948; Greiss and Anderson,1970), which has been suggested to be a result of hormone-induced vasodilation of the uterine vasculature (Greiss and Anderson, 1970).
The
7 resultant increased mass of both the uterine endometrium and myometrium as well as increased uterine vascularization is essential for implantation and development of the embryo.
PHYSIOLOGICAL RESPONSES TO PROGESTERONE
Progesterone is commonly referred to as the hormone of pregnancy and is the principal secretory product of the CL
(reviewed by Rothchild, 1981).
It is absolutely crucial for normal fetal survival and development in all mammalian species known to date.
Progesterone stimulates secretory changes in the intrauterine environment that prepares the endometrium for implantation of the blastocyst and promotes development of decidual cells that supply nutrients
to the early embryo
(Auletta and Flint, 1988; Clark and Markaverich, 1988).
In addition, it decreases the frequency and intensity of uterine contractions thereby preventing expulsion of the implanted embryo (reviewed by Clark and Markaverich, 1988).
Capacity of the CL to secrete progesterone and thus to sustain a normal pregnancy is regulated by hormones synthesized and secreted by the hypothalamus, pituitary, placenta and by the CL itself.
BIOSYNTHESIS OF ESTROGEN AND PROGESTERONE
As stated previously, the primary source of estrogen and progesterone synthesis and secretion in the nonpregnant female mammal is the ovarian follicle and CL, respectively.
Synthesis of these steroids encompasses several
factors,
8 namely, follicle maturation, selection and luteinization.
Changes in steroidogenesis associated with folliculogenesis and luteal development are dependent initially, on the amount of follicle stimulating hormone (FSH) available to the maturing follicle, and later the ratio of FSH and luteinizing hormone (LH) during the preovulatory and luteal phase of the cycle.
Steroid hormones are derivatives of cholesterol of either dietary or de novo origin.
Cholesterol in systemic blood is generally transported in the form of low density or high density lipoprotein (LDL or HDL, respectively).
Low density lipoprotein or HDL binds to specific receptors on the surface of the theca and granulosa cell plasma membranes.
The lipoprotein-receptor complex then enters the cell by way of endocytosis, becoming enclosed in a coated vesicle.
Lysosomes
then enzymatically degrade the coated vesicle
containing lipoprotein-receptor complexes thereby liberating free cholesterol.
This free cholesterol is then available for conversion into steroids.
Excess intracellular cholesterol is esterified and stored in lipid droplets for future use.
Cholesterol can also be synthesized de novo from small
molecule precursors such as acetate.
This pathway of synthesis is activated when cells are deprived of LDL or HDL.
Cholesterol is transported to the mitochondria where it is converted to pregnenolone by the cytochrome P - 45O complex
(Ichii et al., 1963; Roberts et al., 1967).
Pregnenolone is then converted to progesterone by 38-hydroxysteroid
9 dehydrogenase in the smooth endoplasmic reticulum and progesterone or subsequent metabolites of this steroid are secreted into the extracellular space to ultimately enter the blood stream (Miller and Turner, 1963;
Niswender and Nett,
1988) .
Ovarian steroid synthesis and secretion is dynamically regulated by the anterior pituitary gonadotropins FSH and LH.
(Kaltenbach et al., 1968b; Hixon and Clegg, 1969;
Karsch et al., 1971; Denamur et al., 1973; Niswender et al., 1976).
These protein hormones regulate the biosynthesis and secretion
of steroids in the ovary by binding to
high-affinity, low capacity plasma membrane receptors.
The formation of the
hormone-receptor complex activates the enzyme adenylate
cyclase which in turn catalyzes the formation of cyclic
adenosine-3'5'-monophosphate
(cAMP) from adenosine triphosphate (ATP) (Marsh, 1975).
Cyclic AMP then mediates the activation of specific protein kinases
(Ling and Marsh,
1977), which phosphorylate enzymes or other proteins necessary
for the conversion of cholesterol into steroids via the
cholesterol/steroid biosynthetic pathway (Caron et al., 1975;
Caffrey et al., 1979).
Ovarian follicles consist of two distinctly different cell types.
predominant and
Theca cells, which are
arranged along the outer surface of the basement membrane of the follicle, and granulosa cells that lie along the inner surface of the basement membrane.
The interaction of
10 pituitary gonadotropins with the theca and granulosa cells of
the follicle have formed the basis of the two cell-two
gonadotropin model of ovarian steroidogenesis (Falck, 1959;
Short, 1962; Liu and Hsueh, 1986).
During the early follicular stage of development ovarian thecal cells contain
LH but no FSH receptors (Zeleznik et al., 1974; Carson et al.,
1979).
Luteinizing hormone is responsible for stimulating the conversion of cholesterol to pregnenolone in the theca cells
by way of activating the rate limiting cytochrome P-450
cholesterol side chain cleavage mitochondria (Evans et al., 1981).
enzyme in
(P-450scc)
Luteinizing hormone also promotes the conversion of pregnenolone to progesterone in the theca cell endoplasmic reticulum by activating 3Bhydroxysteroid dehydrogenase (38-HSD) (Ireland, 1987).
Progesterone is then converted to the androgens, androstenedione and testosterone, through the activation of
17,20 lyase and 17B-dehydrogenase enzymes, respectively (Evans et al., 1981).
The lipid soluble androstenedione and testosterone can enter the systemic circulation and bind to carrier protein globulins or they can freely diffuse from the theca to the granulosa cell and be converted to estrogens by the granulosa cell aromatase enzyme (Baird, 1977; Dorrington and Armstrong, 1979; Leung and Armstrong, 1980).
In contrast, prior to the Graafian follicle stage of development, granulosa cells have only FSH receptors.
Follicle stimulating hormone is the gonadotropin responsible for stimulating the conversion
11 of cholesterol to progesterone in the granulosa cells.
Like
LH,
FSH activates protein kinases through stimulation of
adenylate cyclase.
In addition to stimulating the synthesis of enzymes necessary for converting cholesterol to progesterone, the FSH-stimulated protein kinases within the granulosa cell activate an aromatase enzyme that catalyzes the conversion of androgens to estrogens (Fortune and
Armstrong, 1978).
Estrogens can in turn act back on the
follicle to stimulate further cell proliferation as well as induce or inhibit (depending on the feedback pathway) the synthesis of more gonadotropin and steroid receptors.
STEROID HORMONE TRANSPORT
Steroid hormones are,
for the most part, transported
throughout the body bound to serum proteins.
However, a relatively smaller fraction may circulate in an unbound or free form.
More specifically, estrogens and androgens are bound to sex steroid hormone binding globulin (SHBG), while progesterone is bound to progesterone binding globulin (PBG).
Both of
these binding globulins
are comparatively high affinity, low capacity carriers.
Alternatively, these steroids may also circulate throughout the body bound to low affinity, high capacity serum albumins.
Only approximately
2% of steroids actually circulate in the free form.
The mechanism by which the steroid is able to dissociate from its carrier and thus freely diffuse through the plasma membrane
12 of the target cell is unclear.
However, Avvakumov et al.
(1986) found the steroid binding globulin in complex with the plasma membrane of human decidual endometrial tissue.
These investigators hypothesized that the binding of the protein binding globulin to the plasma membrane may send some kind of a signal which then causes dissociation of the steroid and binding globulin,
thus allowing simple diffusion of the
steroid across the plasma membrane.
It is also conceivable that the entire steroid and binding globulin complex may enter
the cell by way of endocytosis where the steroid is then
liberated by lysosomal degradation of the protein globulin.
STEROID HORMONE RECEPTORS
In any biological system, receptors function by recognizing a specific hormone from all other molecules in the cellular environment and then upon binding of the hormone, transmit a signal thereby creating a biological response. The magnitude of a hormonal response depends on at least three factors; these include the hormone concentration, the number of available receptors and the affinity of the hormone for the receptor.
Steroid hormone receptors belong to a family of regulatory proteins whose ability to control gene expression is dependent on the binding of their ligand.
Binding of the hormone to its receptor stimulates the synthesis of specific proteins by altering the rate of transcription of specific genes (Mueller et al., 1957; Norman and Litwack, 1987).
13
Over the years, the cellular localization of the steroid hormone receptors has been a source of controversy.
It was once thought and accepted that the unoccupied steroid receptor was localized in the cytoplasmic fraction of the target cell
(Gorski et al., 1968; Jensen et al., 1968).
Upon binding of the receptor to its specific hormone the extranuclear steroid hormone-receptor complex was believed to be translocated to the nuclear compartment where it attached to the DNA to exert stimulatory effects on gene transcription (Gorski et al.,
1968; Jensen et al., 1968).
The foundation for this central
dogma was based on studies in which cells not previously
exposed to a given steroid were homogenized and the nuclear and cytosolic fractions separated by means of differential centrifugation.
After incubation of these separate fractions with a radiolabeled steroid the
steroid receptors were
predominately found in the cytosol.
However, when cells
previously exposed to a radiolabeled steroid were homogenized and separated by centrifugation, the bound steroid receptors were found in the nuclear fraction.
Recently, studies by independent laboratories revealed that occupied as well as unoccupied steroid receptors resided predominantly in the nucleus. Results of these studies indicated that the presence of steroid receptors in the cytosolic fraction of cells as detected in earlier studies was an artifact brought about by their extraction from the nucleus after cell breakage
(Welshons et al., 1984).
14
With the recent availability of monoclonal antibodies specific for a number of steroid receptors and the technique of immunocytochemistry, it is now widely accepted that both the unoccupied and occupied steroid receptors are predominately localized in the nucleus (King and Greene, 1984;
Welshons et al., 1984).
King and Greene (1984) found that
treatment of cells or tissues in vivo or in vitro with
[3H]estradiol -178 altered the intensity of staining of the estrogen receptor but not the distribution of the receptor.
After passage across the plasma membrane of its target cell, the lipophilic steroid hormone may or may not bind to low affinity binding sites in the cytoplasm (Mercer et al., 1981).
The steroid migrates through the cytoplasm and diffuses across the nuclear envelope where temperature dependent noncovalent binding to its specific unoccupied receptor occurs resulting in the formation of the activated steroid-receptor complex
(Miller et al., 1985; Greene and Press, 1986).
The steroid-
receptor complex undergoes a conformational change which stimulates an increase in its affinity for chromatin and
becomes tightly associated with nuclear acceptor sites on the chromatin.
With respect to the estrogen receptor, ligand binding and activation may involve the dimerization of two identical estrogen receptor subunits.
The dimerization may be critically involved in the mechanism by which the estrogen receptor complexes promote changes
(Sabbah et al., 1989).
in gene transcription
The binding of the steroid-receptor
15 complex to the chromatin may cause an unwinding of a specific portion of the DNA strand thus facilitating the binding of ribonucleic acid (RNA) polymerases, which stimulate transcription of specific genes into messenger RNA (mRNA)
(reviewed by Yamamato, 1985).
The mRNA's then translocate to the cytoplasm where they bind to ribosomes and are translated into specific proteins such as enzymes, receptors, low affinity binders and protein hormones, which in turn facilitate the cellular and metabolic effects on the target tissue characteristic of the actions of the steroid hormone in question.
Interestingly, recent immunocytochemical (Berthois et al., 1986) and fluorescence (Parikh et al., 1987) studies have revealed the presence of steroid receptors in the cytoplasm of steroid target cells.
Yamamoto (1985), explained this occurrence by hypothesizing that the nuclear and cytoplasmic distribution of steroid receptors may depend solely on genomic sites.
Because of the enormous amount of DNA that exists in mammalian nuclei this equilibrium would naturally favor that of a
nuclear distribution of receptors
in
intact cells
including the unbound receptors. Thus the majority of unbound
receptors would reside in the nucleus while only a minor
fraction of the free receptors would be transiently cytoplasmic.
These receptors could bind incoming hormone and translocate to the nucleus where modulation of gene expression would then take place.
16
Estrogen and progesterone receptor concentrations are regulated by both estrogen and progesterone.
Specifically, estrogen stimulates the synthesis of
both estrogen
and progesterone receptors thereby enhancing the sensitivity of target tissues to each of these hormones (Zelinski et al.,
1980; DeHertogh et al., 1986; Ekka et al., 1987).
On the other hand, progesterone causes a down-regulation of both progesterone and estrogen receptors (Koligian and Stormshak,
1977b; West et al.,
1986; Selcer and Leavitt, 1988), by interfering with the replenishment or de novo synthesis of the receptor (Hsueh et al., 1976; Pavlik and Coulson, 1976).
17
THE ESTROUS CYCLE
FOLLICULAR PHASE
During the follicular phase of the estrous or menstrual cycle, FSH and LH-induced maturation of the follicle increases the production and release of estrogens into the blood (Karsch et al., 1979; Hansel, 1983).
Just prior to ovulation high levels of estrogen positively feedback to activate the release of gonadotropin-releasing hormone (GnRH) from the hypothalamus
(Karsch et al., 1979).
This in turn stimulates the release of LH and FSH from the pituitary gland.
In the ewe, estradiol secretion begins to rise as the follicle develops and matures.
An increase in estradiol
production of 5 to 10-fold then occurs over the next
few days.
Estradiol peaks at the beginning of the preovulatory LH surge and then rapidly declines to basal
levels (Hauger et al., 1977).
Some researchers suggest that a second estradiol peak occurs in cows (Glencross et al.,
1973; Hansel et al., 1973), and ewes
(Cox et al., 1971).
It is presumed this second low amplitude peak of estrogen stems from renewed follicular development in preparation for a new cycle.
To date, the occurrence and source of the second estradiol peak remains equivocal.
A surge of LH, along with increased levels of FSH precipitate ovulation.
Initiation of the preovulatory LH surge
and subsequent ovulation requires high frequency, low amplitude
18
LH pulses.
These pulses stem from the rising levels of
estrogen that act on the hypothalamus to speed up a GnRH pulse generator (reviewed by Karsch, 1987).
The frequent, estradiol-enhanced GnRH pulses during the follicular phase stimulate the LH surge and ovulation.
Subsequent to ovulation, estrogen secretion declines and LH then stimulates the transformation of the ruptured follicle into a CL as the
thecal and granulosa cells of the follicle undergo rapid
mitosis and hypertrophy.
LUTEAL PHASE
The main function of the CL is to secrete progesterone in order to establish the intrauterine environment necessary for successful implantation of an embryo and maintenance of
early pregnancy in the ewe, mare and primate (Casida and
Warwick, 1945; Ginther and First, 1971).
The CL is the major source of progesterone for pregnancy maintenance to term in the rat, cow, sow and goat (Bazer et al., 1982).
Like the follicle, the CL is composed of two cell types; small and large luteal cells.
Small
luteal cells arise from the
luteinization of theca cells and large cells are derivatives of luteinized granulosa cells (Lemon and Loir, 1977; Uresley and Leymarie, 1979; Rodgers and O'shea, 1982; Niswender et al., 1985).
In the cow (Donaldson et al., 1965), ewe (Kaltenbach et al., 1968), mare (Ginther, 1979) and primate (Moudgal et al.,
19
1971; Mais et al., 1986) LH is the primary luteotropin for maintaining continued progesterone secretion from the CL.
This was demonstrated when administration of LH to cows on day
5, 10,
15 and 20 of the estrous cycle (Schomberg et
al.,
1967), or ewes on day 9 (Niswender et al., 1976) increased the synthesis and secretion of progesterone.
In the ewe, systemic concentrations of progesterone increase from day 3-4 (first day of detected estrus = day 0) to a maximum by day 7-8 of the estrous cycle.
Rising levels of progesterone from the CL block the preovulatory LH surge by suppressing the frequency of the GnRH pulse generator and thereby reduce the frequency and amplitude of LH secretory pulses from the pituitary gland
(Yen et al., 1972;
Foster et al., 1975; Baird, 1978).
Progesterone levels remain high until day 14-15 of the cycle, after which time they decline to basal levels 1-2 days prior to the ensuing estrus as a new cycle is initiated (Baird et al.,
1976; Pant et al., 1977).
Concomitant to declining estrogen and increasing progesterone levels during the luteal phase, an increase in the follicular protein hormone inhibin occurs.
Inhibin feeds back at the level of the hypothalamus and pituitary inhibiting further FSH release (Schwartz and
Channing, 1977).
Just as gonadotropins and steroid hormones interact in dynamic synchrony with one another, so to is the complexity of interaction between ovarian steroids and uterine endometrium during the estrous cycle and pregnancy (Stone et
20 al., 1978).
LUTEOLYSIS
In the absence of fertilization of an ovum
from the ruptured follicle, many laboratory and domestic animals will continue to exhibit estrous cycles.
In most non-primate mammals such as the rat, guinea pig, ruminants and sow, the uterus exerts a local luteolytic action on the ipsilateral corpus luteum of the non-pregnant animal to bring about luteal regression and decreased progesterone secretion (Niswender et al., 1985).
This first became evident when excision of the uterus was found to prolong luteal maintenance in the sow (du
Mesnil du Buisson, 1961), guinea pig (Fischer, 1965), pseudopregnant rat (Barley et al., 1966) and ewe (Inskeep and
Butcher, 1966).
Upon completion of a previous cycle
and initiation of a new cycle, the uterine endometrium secretes the luteolysin prostaglandin Fla
(McCracken et al., 1970;
Goding, 1974).
The secretion of prostaglandin Fla (PGF2a) is under the influence of ovarian progesterone, estradiol -178, and oxytocin (Baird, 1978; Sheldrick et al., 1980).
In the ewe, PGF2a levels increase from day 12 of the cycle, reaching maximum concentrations 1-2 days prior to the subsequent estrus
(Baird et al., 1976).
This luteolytic factor enters the ovary by diffusing across the wall of the uterine vein into the ovarian artery.
The close anatomical proximity of the uterine veins with the convoluted ovarian artery permits the diffusion
21 of substances such as PGF2a directly into the ovarian artery by a countercurrent diffusion mechanism. They thus bypass the general circulation and avoid rapid destruction in the lungs
(McCracken et al., 1970).
Once in the ovary PGF2a targets the progesterone secreting luteal cells of the corpus luteum to cause luteolysis (McCracken et al., 1970).
The mechanism by which PGF2a exerts its luteolytic action on the CL is not known. However, Niswender et al.
(1975)
suggest that the
effects of PGF2a may be via a vascular component because a significant decrease in capillary blood flow
to the CL is
associated with, but does not precede luteolysis.
Thus, it is doubtful that PGF2a causes luteal regression by interfering with blood flow to the CL.
The protein hormone oxytocin is currently believed to
also play an essential
role in the events
leading to
luteolysis (Mitchell et al., 1975; Flint and Sheldrick, 1986).
Uterine PGF2a exerts its effect in a local manner, targeting the ipsilateral ovary and thus causing both inhibition of luteal progesterone secretion and stimulation of luteal oxytocin release.
This was demonstrated by Walters et al.
(1983) and Sheldrick and Flint, (1983) who found that oxytocin secretion was stimulated by an intramuscular injection of the
PGF2a analog, cloprostenol in ewes.
Similiarly,
Schallenberger et al.
(1984) found the same phenomenon to occur in cows.
In ewes, oxytocin triggers the release of more uterine PGF2a and the positive feedback cycle continues until
22 luteolysis is complete (Mitchell et al.,
1975; Flint and
Sheldrick, 1986).
Oxytocin concentrations in both ovine
(Sheldrick and Flint, 1983) and bovine (Abdelgadir et al.,
1987) luteal tissue is maximal during the early luteal phase.
Stormshak et al.
(1969) found that injection of exogenous estradiol into ewes on days 10-11 of the estrous cycle caused premature luteal regression.
It is believed that estradiol also acts to increase uterine PGF2cf secretion thereby promoting luteal regression (Ford et al., 1975).
The mechanism involved in regression of the primate CL is not completely understood.
Shutt et al. (1976) found that removal of the primate uterus did not prolong the maintenance of the CL.
It is believed that an intra-ovarian prostaglandin may be involved, because the ovary is a significant source of
PGF2cc in primates (Shutt et al., 1976).
In primates, the reduction in progesterone secretion that occurs with luteal regression precipitates atrophy of the endometrial epithelium and
invasion of the endometrium by
lymphocytes, causing stromal degradation, exposure of arterioles and mensus, or shedding of the uterine endometrial lining (Knobil,
1973).
With a decrease in both progesterone and ovarian secretion of inhibin, GnRH and the gonadotropins FSH and LH are released and a new menstrual cycle is initiated.
23
EARLY GESTATION
In order to ensure normal embryonic development
and differentiation a complex communication network between the embryo and maternal tissues must be established.
During the preimplantation or preattachment stage of gestation conceptus
secretory proteins and steroids as well as hormones of
maternal origin act upon the uterus and ovaries to ensure maintenance of pregnancy.
In particular, factors of conceptus
origin are important for stimulation of vasodilation
and angiogenesis, which are necessary in order to increase uterine blood flow, stimulate transfer of nutrients into the uterine lumen, protect the conceptus from immunological rejection, and maintain luteal function.
MATERNAL RECOGNITION OF PREGNANCY
Moor and Rowson (1966b) found that the critical period for maternal recognition of pregnancy in the ewe was day 13 of gestation.
Maternal recognition of pregnancy occurs when the presence of an embryo negates the luteolytic action of the uterus on the CL prior to trophoblastic attachment
(Short,
1969).
It was determined that this antiluteolytic action occurred locally, as demonstrated by the fact that a viable conceptus, confined to one uterine horn was able to maintain only the ipsilateral corpus luteum in ewes (Moor and Rowson,
24
1966b) .
Following fertilization and implantation, an endocrine signal, presumably from the conceptus, maintains the function of the CL by blocking the release of PGF2a from the uterus so that progesterone secretion continues (Northey and French,
1980; Fincher et al., 1986).
In many mammals, including the rat, sow, cow and goat, continued secretion of progesterone from the CL is essential for the maintenance of pregnancy to term (Bazer and First, 1983).
In other mammals, such as the human, mare and ewe, the CL is
the primary source
of progesterone initially, but later in gestation (day 56, day
200 and day 60 in the human, mare and ewe, respectively) the placenta produces progesterone or progestins necessary for intrauterine maintenance of the embryo (Ginther and First,
1971; Bazer and First, 1983).
Godkin et al. (1984b) found
that proteins released by cultured day 15-16 conceptuses
prolonged luteal maintenance when introduced into the uterine lumen of cyclic ewes.
One protein involved in the maternal
recognition of pregnancy in the ewe is ovine trophoblast
protein-1 (Godkin et al., 1984a; Anthony et al., 1988).
Ovine trophoblast protein-1 (oTP-1) is a major polypeptide synthesized by the ovine conceptus between days 13 and 21 of gestation.
It directly acts on the uterine endometrium to suppress uterine PGF2a release thereby extending luteal maintenance.
This was demonstrated when oTP-1 infused into the uterine lumen of ewes prolonged the life span of the CL
25 by reducing the production of uterine PGF2a
(Godkin et al.,
1984a).
Interestingly, oTP-1 and the antiviral, immunoregulatory factor a-interferon have been found to be structurally similar (Farin et al., 1990).
Both oTP-1 and ainterferon in fact bind to endometrial a-interferon receptors
(Stewart et al., 1987).
However, it is presently unknown if oTP-1 also plays an immunoregulatory or antiviral role during gestation.
It is conceivable that oTP-1 constitutes just one complex, and that other conceptus secretory proteins make up the remainder of this complex (Martal et al.,
1979; Klopper,
1983).
A functionally similar and structurally
related antiluteolytic bovine trophoblast protein (bTP-1) also exists in cattle (Knickerbocker et al., 1986; Anthony et al., 1988).
Prostaglandins may also play a role in mediating the
local endometrial vascular response and transformation of stromal to decidual cells, both of which are necessary for embryonic development.
Synthesis of prostaglandins by the endometrium, as well as by the blastocyst, has been reported for the mouse (Chepenik and Smith, 1980), rabbit (Harper et al., 1983), ewe (Hyland et al., 1982) and human (Tsang and
Ooi, 1982).
Uterine PGF2a as previously discussed, is responsible for luteal regression (Ford, 1982) during the estrous cycle.
However, uterine and embryonic secretion of prostaglandin E2 (PGE2) occurs during early gestation (Marcus,
1981;
Silvia et al.,
1984).
Prostaglandin E2 (PGE2) is vasodilatory and non-luteolytic, and may be responsible for
26 luteal resistance to the luteolytic effect of PGF2a.
Prostaglandin E2
has been demonstrated to protect luteal
tissue from the actions of PGF2cz both in vivo and in vitro.
Treatment of ewes with PGE2 delayed luteal
regression in nonpregnant ewes (Pratt et al., 1977), and increased progesterone secretion by large luteal cells
(Silvia et al.,
1984).
It has been suggested that oTP-1 may induce endometrial synthesis and secretion of PGE2 and thus prevent luteolysis.
IMPLANTATION OR ATTACHMENT
Among the most critical stages of pregnancy is implantation or in the case of domestic animals, attachment.
Because the conceptus of the sow, cow and ewe has a non-
invasive trophoblast placentation, and diffuse epitheliochorial
the focus of this discussion will be
with respect to attachment rather than implantation of trophoblast unless otherwise indicated.
In ewes the blastocyst attaches
on day
16 of gestation.
Progesterone
is essential for
implantation/attachment in most species.
In species such as
the mouse and rat,
(species that experience a phase of
embryonic diapause or delayed implantation when the mother is lactating), estrogen has been found to be a requirement for implantation (Aitken et al., 1977).
Successful implantation in these species requires an estradiol-primed uterus during proestrus to up-regulate estrogen and progesterone receptors
27
thus sensitizing the uterus to these hormones.
This is
followed by progesterone conditioning during the first 4 days of gestation in order to inhibit myometrial contractility and embryo expulsion.
A preimplantation estrogen peak occurs on day 4
to establish the endometrial vascular
permeability necessary for implantation and nutrient transport.
As pregnancy progresses secretion of progesterone again increases and then remains relatively constant until parturition
(Nalbandov, 1971; Aitken, 1979).
In species such as the sheep
(Cumming et al., 1974),
which do not exhibit embryonic
diapause, progesterone alone will allow attachment to occur if the proliferative effects of estrogens on the endometrium have already been achieved.
In order for successful attachment to occur in the ewe, the intrauterine environment must be primed with estrogen secreted during estrus.
This steroid enhances endometrial and myometrial vascularization and proliferation of various cell types in the uterus (Moor and Rowson, 1959; Bindon,
1971; Miller and Moore, 1976).
Progesterone can function successfully if cells of the
reproductive organ have been exposed to estradiol,
which induces the formation of progesterone receptors (Muldoon,
1981).
Together with estradiol and the action of anterior pituitary hormones on the CL, progesterone acts to transform the uterine endometrium from a proliferative to a secretory state.
Subsequent to estrus, serum concentrations of progesterone increase as the CL develops accompanied by a
28 decline in serum concentrations of estrogen (Moor and Rowson,
1959; Bindon, 1971;
Miller and Moore,
1976).
Although estrogen is not obligatory for implantation in some species, it has been found to promote increased placental development
(Dalton and Knight, 1983) and litter size (Wilde et al., 1976) in gilts.
Preimplantation blastocysts in the gilt or sow
(Fischer et al., 1985)
are able to convert androgens and
progestins to estrogens (Perry et al., 1973; Gadsby et al.,
1980; Fischer et al., 1985), which are essential for migration and spacing of embryos within the uterine horns.
In addition, estrogen of conceptus origin ensures maintenance of the CL because in the gilt this steroid is luteotropic (Gardner et al., 1963).
Because of this phenomenon, it is presumed that estrogen of maternal origin is not necessary for attachment of embryos in this species.
It has been demonstrated that significant amounts of estrogens are present in the systemic circulation prior to embryo attachment in the cow (Eley et al., 1979; Shemesh et al., 1979), ewe (Ellinwood, 1978;
Reynolds et al., 1982) and mare (Zavy et al., 1979).
However, questions remain about the origin of these estrogens in the indicated species.
Steroid metabolizing enzymes such as 38-hydroxysteroid dehydrogenase, 1713 -hydroxysteroid dehydrogenase and aromatase have been detected in rabbit, mouse, rat and hamster preimplantation blastocysts (George and Wilson, 1978; Singh and Booth, 1979; Wu, 1985) establishing the possibility for
29 embryonic steroid synthesis.
Evidence which supports the concept that the preimplantation embryo is able to synthesize and metabolize steroids is complicated by the fact that some species (rat and mouse) require maternal estrogen for implantation while other species (pig, sheep, rabbit, hamster and guinea pig) require only maternal progesterone.
These differences among species may exist because rat and mouse blastocysts are unable to supply sufficient estrogen compared with those of the pig, sheep, rabbit, hamster and guinea pig.
Once established, the fetoplacental unit is able to metabolize and interconvert steroids, and thus possesses the ability to potentially influence events such as uterine secretory activity, nutrient transport and uterine blood flow (Singh and
Booth, 1979; Wu, 1985).
30
SEASONAL REPRODUCTION
Reproduction in some species of animals is regulated by environmental cues such as availability of food or photoperiod.
Animals whose reproductive activity coincides with changes
in photoperiod are commonly referred to as
seasonal breeders.
Traditionally these animals have been referred to as long- or short-day breeders but in actuality these designations denote the hours of daylight to which the animal is exposed.
Changes in circannual photoperiod perceived by the animal are transduced into neuroendocrine
signals that ultimately effect gonadal
activity.
This photoperiod-induced regulation of reproductive activity ensures, at least in part, that parturition occurs at a time
when environmental factors are most beneficial to
permit survival of newborn offspring.
Species such as hamsters, ferrets, mink, horses and birds are long-day breeders, with mating occurring during the spring and summer (Gwinner and
Dorka,
1976; Thorpe and Herbert, 1976).
Sheep, deer, and certain other species of wildlife are short-day breeders and mate during the fall or winter (Karsch et al., 1984; Bittman et al., 1985).
31
MELATONIN
Melatonin is a hormone whose secretion is characterized by circadian and circannual rhythms
(Karsch et al., 1984).
This hormone
is
believed to play an essential role
in regulating seasonal reproduction.
Melatonin secretion is maximal during darkness.
Thus, duration of secretion, and hence, daily quantity of melatonin secreted, is greater during
the seasons of the year when hours of daylight are the
shortest (fall and winter).
In essence, the circadian rhythm of melatonin secretion synchronizes the circannual rhythm of reproduction.
The mechanism by which photoperiodic signals regulate melatonin secretion involves various components of the central nervous system.
Light entering the eye stimulates the retina to produce neurotransmitters,
which in turn activate the
retinohypothalamic nerve fibers that terminate in the suprachiasmatic nucleus (SCN) of the hypothalamus.
From the
SCN,
nerves descend through the lateral hypothalamus to
synapse in the superior cervical ganglia with post-ganglionic autonomic nerves that terminate in the pineal gland.
The stimulus evoked by light suppresses the release of a neurotransmitter (norepinephrine) in the pineal gland (Moore and Klein, 1974; Stetson and Watson-Whitmyre, 1976; Karsch et al., 1984).
Absence of light results in the release of this neurotransmitter, which then binds to
B-adrenergic receptors
32 located in the plasma membrane of pinealocytes.
Binding of norepinephrine to its receptor promotes activation of adenylate cyclase, which catalyzes the conversion of ATP to cAMP.
This nucleotide then binds to protein kinase to activate its catalytic subunit which then stimulates the
activity of N-acetyltransferase, the rate limiting enzyme involved in the synthesis of melatonin.
In species such as sheep, melatonin is believed to act back upon the hypothalamus to regulate the pulsatile secretion of LH by modulating the frequency of the gonadotropin releasing hormone (GnRH) pulse generator.
This action of melatonin is accomplished by
sensitizing the GnRH pulse generator to the feedback effect of estrogen (Karsch et al., 1980; Goodman et al., 1982; Karsch et al., 1984).
Anestrus in most breeds of sheep in the northern hemisphere begins during late winter and extends through late
August or early September.
This reproductively quiescent period is characterized by a comparatively low daily production of melatonin (due to extended daylight) and reduced secretion of ovarian estradiol.
The limited daily production
of melatonin presumably decreases the sensitivity of the
hypothalamic GnRH pulse generator to the feedback action of estradiol.
Hence, the pulsatile release of GnRH is suppressed resulting in a corresponding reduction in LH secretion.
As hours of daylight decrease during late summer and fall the increased daily production of melatonin increases the
33 sensitivity of the hypothalamus to the feedback action of estradiol. Consequently, increased activity of the GnRH pulse generator stimulates increased frequency of LH secretion with a resultant stimulation of additional estradiol production by the ovarian follicles. This hypothalamic-hypophyseal-gonadal interrelationship ultimately leads to the preovulatory surge of LH secretion, which induces the first ovulation of the breeding season.
In contrast, the pineal gland of long-day photoperiodic breeders such as the hamster and ferret, secretes low levels of melatonin during the breeding season and high levels of
the hormone during the anestrous period.
In this case
melatonin is inhibitory to the hypothalamic secretion of GnRH and the subsequent secretion of gonadotropins, thus causing a decrease
in ovarian steroidogenesis and inhibition of
ovulation.
Melatonin infusions into male hamsters equally induced gonadal atrophy in both sham and SCN-lesioned animals; demonstrating that the SCN may not be an essential component of the neural circuits that measure the melatonin signal in long-day photoperiod breeders (Maywood et al., 1990).
Prolactin, another non-ovarian, seasonally regulated
hormone may also
be involved
in mediating the pivotal
neuroendocrine changes that occur during the breeding and nonbreeding season. In fact, secretion of melatonin and prolactin
have been shown to be inversely related
in a number of
species.
Melatonin inhibits prolactin release (Clark, 1980;
34
Glass and Lynch, 1983), as demonstrated by an increase in prolactin secretion after pinealectomy.
PROLACTIN
Prolactin secretion is depressed in hamsters (long-day breeders) exposed to shortened hours of daylight and exogenous administration of this hormone can induce gonadal growth and steroidogenesis.
This effect of prolactin may be due to an increase in LH receptor numbers (Holt et al., 1976; Advis et al., 1981).
Of particular interest with respect to this
thesis is the fact that the secretion of prolactin has been
demonstrated to
be influenced
by photoperiod
in goats,
(Buttle, 1974), hamsters (Bex et al., 1978), heifers (Schams and Karg, 1974; Peters and Tucker, 1978) rats (Williams et al., 1978)
and ewes
(Posner et al., 1974;
Webster and
Haresign, 1983;
Kennaway et al., 1983).
In general, a positive correlation between increasing daylight and serum prolactin was detected in each of these species.
Because secretion of prolactin and melatonin occurs in response to changes in photoperiod it is
conceivable that they are
necessary components in regulating seasonal reproduction.
Prolactin is a protein hormone (MW=24,000) whose structure and activity are similar to those of growth hormone.
Prolactin is secreted from the lactotropes of the adenohypophysis (Nicoll, 1974). Interestingly, prolactin mRNA is found in many tissues throughout the body indicating a
35 potential for synthesis.
However, in many of these tissues, synthesis, secretion and the action of this hormone are at present unknown.
The classical function of prolactin is to promote the development of the mammary gland during gestation and postpartum lactation (Nicoll, 1974).
This stimulation is essential for milk production and ultimate survival of offspring.
However, prolactin may also play far reaching and essential roles in regulating the reproductive cycle of many mammals through its influence on gonadotropic and steroidogenic responses.
In fact, the presence of prolactin receptors found widely distributed in a number of non-mammary tissues from several different species (Posner et al., 1974;
Rolland et al.,
1976;
Rose et al.,
1986) indicates the
potential diversity of actions this hormone may possess.
During pregnancy, prolactin can also be found in the amniotic fluid and extraembryonic membranes of the developing fetus as well as the maternal endometrium (Riddick, 1985).
The interaction of prolactin with its specific target cell receptor triggers a cascade of molecular processes culminating in a series of diverse responses, including, the synthesis of its own receptor (Djiane and Durand, 1977; Huhtaniemi and
Catt, 1981; Muldoon, 1981).
However, the sequence of molecular events by which prolactin expresses its action on target cells is not completely understood.
Estrogen stimulates the synthesis and release of
36 prolactin in rabbits (Tindal, 1969), rats (Neill, 1974; Brann et al., 1988) and women (Vekemans and Robyn, 1975).
It is therefore not surprising that prolactin concentrations are greatest during proestrus, estrus, the end of gestation, and early lactation when estrogen levels are the highest (Sar and
Meites, 1968;
Swanson et al.,
Webster and Haresign, 1983).
1972;
Boyer et al.,
In turn,
1975;
it appears that
prolactin inhibits estrogen production.
This occurrence has been observed in several species including the rat and mink
(Wang et al., 1980; Dorrington and Gore-Langton, 1981; Wang and Chan, 1982; Slayden, 1990).
One explanation of this
phenomenon as demonstrated by Tsai-Morris et al.
(1983) is the ability of prolactin to prevent estrogen synthesis in rats by inhibiting granulosa cell aromatase activity.
Inhibition of aromatase by prolactin may be due to the ability of the hormone to stimulate production of intracellular factors that suppress the activity of the enzyme.
Alternatively, such factors may interfere with the ability of gonadotropins to maintain normal enzyme activity.
Interestingly, Chilton and
Daniel (1987) found that treatment of ovariectomized rabbits with prolactin caused an increase in the uterine endometrial surface area as well as stimulation of both uterine estrogen and progesterone receptors.
Prolactin plays an essential role in promoting luteal function in rodents (Greenwald and Rothchild, 1968).
In these animals, prolactin is the primary luteotropin responsible for
37
luteinization of granulosa cells necessary for
continued progesterone secretion (Grota and Eik-Nes, 1967).
It is
believed that prolactin accomplishes this role by increasing
LH receptor levels
(Advis et al., 1981)
as well as by
preventing catabolism of newly synthesized progesterone (Advis et al., 1981).
This process enhances the effectiveness of LH to stimulate the steroidogenic biosynthetic pathway.
In addition prolactin also increases the activity of cholesterol
esterase and
3B-hydroxysteroid dehydrogenase,
the enzyme
required for converting pregnenolone to progesterone.
The increased levels of prolactin-stimulated progesterone secretion (Advis et al., 1981)
in turn causes
a downregulation and inhibition of synthesis of progesterone receptors in the rabbit, hamster and mink (Djiane and Durand,
1977; Bex et al., 1978; Slayden, 1990, respectively).
Prolactin has also been implicated in stimulating progesterone secretion in non-rodent species (Advis et al., 1981; Slayden,
1990), however, the mechanism remains unclear.
38
EXPERIMENT 1
INTRODUCTION
There is a paucity of information about the fluctuations
that occur in uterine estrogen and progesterone receptor
concentrations during the estrous cycle and early pregnancy in the ewe.
The presence of a conceptus in one uterine horn ensures maintenance of the corpus luteum in the ipsilateral ovary (Moor and Rowson, 1966a), suppresses the synthesis and secretion of prostaglandin Fla by the uterine endometrium
(Moor and Rowson, 1966b) and undoubtedly alters other aspects of uterine metabolism and function.
However, it has not yet
been determined whether the conceptus influences uterine
estrogen and progesterone receptor concentrations and whether
such an effect of the conceptus would be localized or
systemic.
An understanding of how uterine steroid receptor concentrations vary between pregnant and nonpregnant animals
may provide
insight regarding the role of steroids in regulating luteolysis during
the estrous
cycle
and the
establishment of maternal recognition of pregnancy.
Because embryonic mortality is one of the most prevalent reasons for reduced reproductive efficiency it is important that research be conducted that may explain these
Certainly, inappropriate levels of embryonic losses.
estrogen and(or)
39 progesterone receptors during early gestation could result in desensitization or overstimulation of the intrauterine environment at inappropriate times thereby causing embryonic loss.
The present experiment was conducted to determine whether the presence of a conceptus would alter endometrial concentrations of estrogen and progesterone receptors in the gravid and nongravid uterine horns compared with those present in uteri of nonpregnant ewes.
MATERIALS AND METHODS
Animals
Nine mature Polypay ewes were checked for estrus twice
daily with a vasectomized ram until all had exhibited an
estrous cycle of normal duration (16.3 ± 0.9 days).
On day
10 of a subsequent cycle ewes were anesthetized by intravenous injection of 2% thiamylal sodium and inhalation of a mixture of oxygen and halothane.
A midventral laparotomy was
performed through which the uterus and ovaries were exteriorized.
All ewes were unilaterally ovariectomized by removing an ovary bearing CL, and the ipsilateral (adjacent) uterine horn was ligated with #2 surgical silk, 1 cm distal to the external bifurcation.
Approximately one-third to onehalf of the anterior ipsilateral uterine horn was removed and immediately placed on ice for determination of endometrial
40 concentrations of progesterone and estrogen receptors. Tissue was transported to the laboratory within 90 min of surgical removal.
All ewes were allowed to complete one full recovery estrous cycle and at the ensuing estrus, were bred twice to a fertile ram.
The first breeding occurred at the time of detected estrus (day 0) and the second breeding occurred 8 h later if estrus was first detected in the morning and 12 h later if estrus was first detected in the afternoon.
On day
18 of gestation 10 ml of blood were collected by
jugular venipuncture for subsequent analyses of serum concentrations of estrogen and progesterone.
Ewes were relaparotomized and
the remaining portion of the ipsilateral and the
entire
contralateral uterine horns were removed for analyses of
endometrial estrogen and progesterone receptors.
Pregnancy was verified by the presence of an embryo in saline flushings of the contralateral uterine horn.
Blood samples that were collected prior to surgeries were allowed to clot at room temperature (20 °C) for approximately
4 h and then stored overnight at 4°C.
Blood samples were then centrifuged at 500 x g for 15 min at 4°C and the resultant
sera were decanted into vials and stored at -20°C
until assayed for concentrations of estrogen and progesterone.
Nuclear Estrogen Receptor Assay
Intercaruncular uterine endometrium was carefully
dissected from the
lumen of
the uterine horn.
Unless
41
otherwise indicated, all procedures were performed at 4°C
using acid-washed, siliconized glassware.
Approximately 300
± 5 mg of endometrium were weighed and homogenized with an all
glass tissue grinder in 2 ml of ice cold TE
buffer (0.01M
Tris-0.0015M Na2EDTA, pH 7.4, at 25°C) and centrifuged at 1000
x g for 15 min.
The supernatant containing cytosol was
decanted into a tube and saved for analysis of estrogen
receptor concentrations.
The remaining nuclear pellet was washed three times with cold TE buffer and then resuspended with buffer to an equivalent of 50 mg of endometrium/ml.
The nuclear fraction was vortexed and 1 ml aliquots were pipetted into each of four tubes.
Nuclear concentrations of estrogen receptor were determined by exchange and binding assay as described by Koligian and Stormshak (1977a).
The exchange was performed in duplicate using 6,7[3H]- estradiol -17B (specific activity 1.0 mCi/mmol, NEN Research Products, Boston, MA) at a concentration of 2 x 10-8M for determination of total binding and labeled estradiol -178 plus a 100-fold excess of diethylstilbestrol (DES) for determination of nonspecific binding.
Samples were incubated for 30 min at 37°C.
Following this exchange period, samples were placed on ice for
10 min to terminate the exchange process, and centrifuged for
10 min at 1000 x g.
Nuclear pellets were then washed four
times with 2 ml of cold TE buffer to remove any
unbound ligand.
The nuclear pellets were then extracted with 2 ml of redistilled absolute ethanol overnight at 4°C.
Following
42 extraction, the samples were vortexed, centrifuged
(1000 x g), and the ethanolic supernatants scintillation vials.
were decanted into
Ten milliliters of scintillation fluid
(0.7% 2,5-diphenyloxazole (PPO) and 0.01% p-bis[2-(5phenyloxazoly1)]-benzene (POPOP)
dissolved in toluene and
Triton X-100 (2:1)) were added and samples were then counted.
Cytosolic Estrogen Receptor Assay
The cytosolic supernatant that was collected after
homogenization and centrifugation of uterine endometrium was brought to a volume of 50 mg/ml with cold TE buffer.
Onehundred microliters of dextran-coated charcoal (0.5% dextran:5% charcoal) were added and incubated for 5 min to remove any free ligand.
The sample was centrifuged for 10 min at 1000 x g and the supernatant decanted into a clean tube.
This procedure was repeated a second time to ensure all charcoal had been removed. One milliliter aliquots were pipetted into each of four tubes to which were added 617[3]flestradio1-1713 at a concentration of 2 x
104IM (total binding tubes) or labeled estradio1-178 plus a 100-fold excess of DES
(nonspecific binding tubes).
min at 37°C.
Samples were incubated for 30
After completion of the exchange process, the cytoplasmic fraction was subjected to 500 Al of hydroxylapatite (60% solution v/v of hydroxylapatite in TE buffer) for adsorption and incubated at 4°C for 1 h with 15 min intermittent vortexing.
Following incubation the
43 hydroxylapatite to which the estrogen-receptor complex was adsorbed was washed four-times with 2 ml of TE buffer and then subjected to extraction with 2 ml of redistilled absolute ethanol at 4°C overnight.
After centrifugation the supernatant was decanted into scintillation vials containing
10 ml of scintillation fluid as described above, and counted.
Nuclear Progesterone Receptor Assay
Aliquots of intercaruncular endometrium were homogenized
in ice cold TEM buffer (10mM
Tris-HC1, 1mM Na2EDTA, 12mM monothioglycerol, 30% v/v glycerol, 5mM Na molybdate, pH 7.4
and centrifuged at 1000 x g for 10 min.
The at 25°C), supernatant was decanted into tubes for assay of cytosolic concentrations of progesterone receptors.
The nuclear pellet was washed four-times with TEM buffer and then resuspended to a final volume of 100 mg endometrium/ml of buffer.
Samples were vortexed and aliquots of 1 ml were pipetted into each of four tubes.
The nuclear exchange assay for progesterone receptors was performed as described by West et al. (1986).
The exchange was carried out by adding
[3Hjpromegestone
(17,21-dimethy1-19-nor-4,9-pregnadiene-3,20-dione,alsoknown as R5020, specific activity 1 mCi/mmol, NEN Research
Products,
Boston, MA)
at a concentration of 1 x
10-6M to the total binding tubes. The synthetic glucocorticoid dexamethasone was added (2 x
10-4M) to prevent any progesterone from binding to the glucocorticoid receptors.
Similarly the labeled ligand
44 and dexamethasone were added to non-specific binding tubes as described above with an additional 100-fold excess of unlabeled promegestone.
Samples were incubated overnight at
4°C to complete the exchange process.
After completion of the exchange, the nuclear pellet was washed four-times with TEM buffer and extracted with 2 ml of absolute ethanol at 4°C overnight. Following extraction, the supernatant was decanted into scintillation vials containing 10 ml of scintillation fluid and counted.
Cytosolic Progesterone Receptor Assay
After homogenization and centrifugation, the cytosolic supernatant was also subjected to a progesterone receptor exchange assay.
The supernatant was brought to a final volume of 100 mg equivalent of endometrium/ml of TEM buffer.
Onehundred microliters of dextran-coated charcoal (0.5% dextran:5% charcoal in TE buffer) were added to the supernatant to remove any free ligand, centrifuged (1000 x g) and decanted into new a tube.
This washing procedure was repeated an additional time to ensure that all charcoal was removed from the supernatant.
The cytosolic sample were then aliquoted into 1 ml fractions and
subjected to the same binding exchange procedure as described for the nuclear
fraction.
After the overnight incubation at 4°C, 500 Al of dextran-coated charcoal (0.05:0.5% in saline, pH 7.4) were added.
phosphate buffered
Samples were vortexed at 5 min
45 intervals during a 10 min incubation centrifuged at 1500 x g for 10 min.
period and then
Five-hundred microliters of supernatant were pipetted into scintillation vials containing 10 ml of scintillation fluid and counted.
DNA Determination
Deoxyribonucleic acid concentration in the endometrial tissue were measured using the procedures described by Burton
(1956).
In brief, the resultant nuclear pellet from the exchange assay was stored at 4°C in 0.5M perchloric acid (PCA) until analyzed for DNA.
Standards containing a known amount of calf thymus DNA
(0, 50, 100, 150, 200, 250, 300, 350, 400,
450 and 500 µg /ml dissolved in 5mM sodium hydroxide solution), were pipetted (100 Al) in duplicate.
Bovine serum albumin (1 ml of a 0.1% solution) and 1 ml of 0.5M PCA was then added to the standard tubes to precipitate the protein and DNA.
All tubes were centrifuged at 1000 x g for 10 min after 1 h of 4°C
incubation and
the resultant supernatant was discarded.
Perchloric acid was added to all tubes (2 ml of 0.5M
PCA)
which were then capped with marbles and placed in a 90°C
water-bath for 30 min in order to hydrolyze the DNA.
Tubes were then cooled at 4°C for 30 min and subsequently centrifuged at 1000 x g for 10 min.
The resultant supernatant was pipetted (500 Al) into fresh tubes and colored for 16 h with 1 ml of diphenylamine reagent (1.5 g diphenylamine dissolved in 1.5 ml of 96% concentrated sulfuric acid and 100
46 ml of concentrated glacial acetic acid).
Samples were then
read using a spectrophotometer set at 600 Am wavelength.
Concentration of estrogen and progesterone receptors were based on radioactive counts converted to fmol/Ag DNA.
Serum Progesterone Radioimmunoassay
Serum concentrations of progesterone according to procedures described were measured by Koligian and Stormshak
(1977a).
In brief, duplicate 100 Al aliquots of serum were extracted with 2 ml of benzene:hexane (2:1 v/v) by vortexing.
Separation of organic solvent from the serum was accomplished by subjecting tubes to -20°C until the serum was frozen and then decanting the benzene:hexane into tubes.
Subsequently the benzene:hexane was evaporated using a gentle stream of air.
One-hundred microliters of progesterone antibody (#337 antiprogesterone-11-BSA,
Gordon Niswender,
University) diluted 1:2400 with 0.1%
Colorado State
gelatin phosphate buffered saline (GPBS), were added to tubes containing the extracted progesterone or quantities of standards (10, 25, 50,
100, 200, 400, 500, 800, 1000 and 2000 pg of progesterone/100
Al)
and allowed to equilibrate for >30 min.
Following equilibration, 100 Al of competitor 1,2,6,7[3H]-progesterone
(12 x 103 dpm; 115 Ci/mmol; New England Nuclear [NEN], Boston,
MA), were added to all tubes and the mixture was allowed to incubate at 4°C overnight.
After overnight incubation 1 ml dextran-coated charcoal (0.25% dextran:2.5% charcoal in 0.01
47
M NaH2PO4(H20)
buffered saline) was added and the samples
incubated at 4°C for 15 min to remove any free
ligand.
Samples were then centrifuged at 1700 x g for 10 min.
The resultant supernatant was decanted into scintillation vials and counted.
The sensitivity of the assay was 10 pg/assay tube (100 Al).
All serum samples for the progesterone
radioimmunoassay were analyzed in a single assay where the intraassay coefficient of variation was 8.7 ± 0.37% and the extraction efficiency was 91.5 ± 3.8%.
Serum Estradiol Radioimmunoassay
Serum estradiol was measured using the extraction
procedures described by Hotchkiss et al.
(1971)
and the
radioimmunoassay described by Butcher (1977).
Briefly, 200
Al of sample serum were pipetted in triplicate into 10 ml centrifuge tubes with screw caps.
Every third tube received
100 Al of
2,4,6,7(3H]- estradiol -178 (diluted to 3500 cpm/200
Al in 0.1% GPBS, NEN) for determination of extraction efficiency.
Anesthetic grade anhydrous diethyl ether (3 ml) was added and samples were vortexed for 30 sec.
In order to ensure complete separation of organic solvent from the serum, samples were centrifuged at 1000 x g for 10 min.
The sample tubes were then snap frozen in a dry ice and ethanol bath.
The resultant ether layer was decanted into 12 x 75 mm tubes and evaporated to dryness under light
air in a 37°C water
bath.
The frozen pellets from the original tubes were allowed
48 to thaw and the above procedure was repeated.
Sample tubes containing the dried extractant were then stored at 4°C until the estradiol RIA was conducted.
Standards containing 0, 0.5, 1, 5, 10, 25, 50, 100, 200 and 500 pg estradiol - 17!3/100 Al redistilled absolute methanol were pipetted in duplicate and dried under light air in a 37°C water-bath while extractant tubes were allowed to come to room temperature.
To all except blank tubes,
100 Al of 0.3%
gelatin phosphate buffered saline (GPBS) was added [0.3% Knox gelatin, 0.01M Na2HPO4(H20), 0.15M NaC1, 0.01% merthiolate, pH
7.0].
Blank tubes received 200 Al of 0.3% GPBS.
Tubes were vortexed and allowed to equilibrate for 15 min in order for
the GPBS to take up the ligand.
All tubes excluding the blanks received 100A1 of antiestradiol -178 (anti-E2hemisuccinate-BSA diluted to a concentration of 1:10,000 in
0.1% GPBS).
In addition, 200 Al of 2,4,6,7[3H)- estradiol -178
(3500 cpm/200 Al) were added to all tubes, blanks included, and vortexed.
Samples were then placed in a 37°C water-bath for 5 min for the binding process to occur.
After incubation tubes were again vortexed and placed in a 4 °C ice bath for 40 min.
Rapidly, 800 Al of ice cold dextran coated charcoal
(1% dextran T-70 from Sigma Chemical, St. Louis, MO, 0.25% Neutral
Norit from Pharmecia Fine Chemicals,
Piscataway, NJ) were added to tubes and vortexed.
After 10 min on ice, tubes were then centrifuged at 2500 x g for 10 min and the resultant
supernatant was decanted into vials containing 10 ml of
49 scintillation fluid and counted.
All serum samples for
estradiol radioimmunoassay were analyzed in a single assay,
with a sensitivity of
2.3 pg/tube.
The coefficient of
variation was 10 ± 0.43% and extraction efficiency was 95 ±
2%.
Statistical Analysis
Data on nuclear and cytosolic concentrations of estrogen and progesterone receptors were subjected to log transformation because of heterogeneity of variances.
The transformed data were analyzed by least squares analysis of variance for an experiment of completely randomized block, repeated measures design. Differences among means were tested for significance by use of Students t-test.
For purposes of presentation, data are presented as nontransformed means.
RESULTS
Endometrial nuclear concentrations of estrogen receptor in uterine horns of ewes on day 10 of the estrous cycle were significantly greater (1.21 ± 0.22 fmol/Ag DNA, p<.01) than in the pregnant and nonpregnant horns of ewes on day 18 of gestation (0.13
±
0.23 and 0.22
± 0.25 fmol/Ag DNA,
respectively; Figure 1).
Similarly, cytosolic concentrations of estrogen receptor in endometrium on day 10 of the estrous cycle were significantly greater
(4.90 ± 0.56 fmol/Ag DNA, p<.01) than in endometrium of pregnant and nonpregnant horns
50 of ewes on day 18 of gestation (1.25 ± 0.58
and 1.10 ± 0.64
fmol/gg DNA, respectively).
Nuclear concentrations of progesterone receptor in
endometrium of ewes on day 10 of the estrous
cycle were significantly higher (0.63
± 0.21
fmol/Ag DNA) than in endometrium of pregnant and nonpregnant uterine horns of ewes on day 18 of gestation
(0.18 ± 0.21, p<.05 and 0.13 ± 0.21
fmol/Ag DNA, p<.01
respectively; Figure 2).
Cytosolic
concentrations of progesterone receptor on day 10 of the
estrous cycle were determined to be 1.14
± 1.03 fmol/Ag DNA.
Data on cytosolic concentrations of progesterone receptor in both the pregnant and non-pregnant horns of ewes on day 18 of gestation were invalidated by excessive nonspecific binding
(NSB) of the ligand (77.5 ± 8.6%).
Serum concentrations of estradiol -1713 on day 18 of gestation as determined by radioimmunoassay were 16.56
± 2.43
pg/ml.
Mean serum concentration of progesterone on day 18 of gestation was 1.74
± 0.57 ng/ml.
6.00
5
,
?
4.80
3.60
INN
NUCLEAR CYTOSOL
2.40
7
N 120
0.00
d 10 OF CYCLE d18 PREG.HORN d 18 NPREG.HOFts1
Fig. 1.
178
Specific binding of [ flestradiolto nuclear and cytosolic estrogen receptors (mean ± SE) in endometria of ewes on day 10 of the estrous cycle and in endometria of gravid and nongravid uterine horns of ewes
on day 18 of gestation.
Mean nuclear and
cytosolic concentrations of estrogen receptors, day 10 vs day 18 gravid and nongravid horns differ (p<.05).
1.00
3.1
0.80
0.60
0.40
0
0
11.
A
0.20
0.00
d10 OF CYCLE d18 PREGHORN d18 NPFEG.HORN
Fig.
2.
Specifically bound [ 4]115020 to nuclear progesterone receptors (mean ± SE) in endometria of ewes on day 10 of the estrous
cycle and in endometria of the gravid and
nongravid uterine horns of ewes on day 18 of gestation.
Day 10
vs day
18
gravid and
nongravid horns differ in concentrations of progesterone receptors (p<.05).
51
52
DISCUSSION
Results of Exp. 1 indicate that the endometrium of the nongravid horn of ewes on day 10 of the estrous cycle contains
greater concentrations of nuclear and
cytosolic estrogen receptors than the endometrium of gravid and nongravid horns
of ewes on day 18 of gestation.
Similiarly, endometrial nuclear concentrations of progesterone receptor were greatest on day 10 of the cycle than in gravid and nongravid uterine
horns of ewes on day 18 of
gestation.
These changes in
uterine estrogen and progesterone receptor are consistent with the reported ability of progesterone to down-regulate the estrogen receptor as well as its own receptor (Koligian and
Stormshak, 1977a; Selcer and Leavitt, 1988).
The greater concentration of these receptors on day 10 of the cycle most likely reflects the gradual change in the concentrations of these receptors that occurs from the time of estrus.
Endometrial concentrations of nuclear and cytosolic estrogen receptors have been reported to be maximal at estrus and to decrease during the luteal phase of
the cycle of the ewe
(Koligian and Stormshak, 1977a; Miller et al.,
1977), and cow
(Zelinski et al., 1982) as progesterone secretion increases.
Few data are available regarding uterine concentrations of steroid receptors during early gestation in ruminants.
However, Senior (1975) reported low levels of uterine cytosolic estradiol binding sites on day 20 of gestation in
53 cows.
Continued production of progesterone by the corpus luteum whose maintenance is ensured by the conceptus, as well as low production of ovarian estrogen may be surmised to be responsible for the reduced concentrations of progesterone and estrogen receptors observed in this study.
Indeed, the low concentrations of estrogen and progesterone receptors observed
in the uteri of ewes on day 18 of gestation most
likely represent the minimal levels of these receptors necessary to
ensure basal function of the uterus.
Logically, greater concentrations of receptor than detected during early gestation, especially for estrogen, would not be consistent with the ability of the uterus to remain in a comparatively quiescent state necessary for maintenance of pregnancy.
It
is possible that some other factor (perhaps of
conceptus origin) besides progesterone may also operate to suppress the uterine concentrations of steroid receptors. This possibility
is supported in part by the observation that
endometrial concentrations of nuclear and cytosolic estrogen and nuclear progesterone receptors did not differ between pregnant and
nonpregnant uterine horns on day 18 of gestation.
These latter data indicate that the release of a conceptus secretory factor may influence uterine concentrations of estrogen and progesterone receptors in a systemic rather than local manner.
This is further supported by the fact that vascular connections between horns were blocked by a ligature at the external bifurcation.
54
Unfortunately, quantification of levels of cytosolic
progesterone receptor concentrations on day 18 of gestation, as determined by receptor exchange assay, was confounded by extremely high nonspecific binding.
One explanation for this phenomenon may be the increase in systemic binding proteins
such as progesterone binding globulin
(PSG) during early gestation.
for
Progesterone binding globulin has a high affinity progesterone and has been shown to increase in concentration during gestation in several species (Westphal,
1983).
Similiarly,
increased levels of systemic binding
globulins that bind estrogens (such as sex steroid hormone binding globulin) may have contributed to nonspecific binding in the assay for estrogen receptor, but to a lesser extent.
It
is conceivable that the problems encountered by high
nonspecific binding could have been circumvented by using a substantially greater quantity of unlabeled ligand in the assay.
These data indicate that the concentrations of estrogen and progesterone receptors during early gestation in the ewe are significantly lower than during the midluteal phase of the estrous cycle when progesterone concentrations are maximal.
Additionally, results of this experiment can be interpreted as suggesting a systemic effect of the conceptus on uterine concentrations of estrogen and progesterone receptors.
55
EXPERIMENT 2
INTRODUCTION
Only recently have researchers identified the hormonal mechanisms by which varying photoperiod functions to control
seasonal breeding of ewes.
In the northern hemisphere,
exposure of ewes to reduced hours of daylight in the fall results in increased duration of melatonin secretion during
the lengthening hours of darkness
(Karsch et al., 1984;
Bittman et al., 1985).
It has been proposed that increased daily secretion of melatonin relieves the sensitivity of the hypothalamic GnRH pulse regulator to the negative feedback action of estrogen (Karsch et al., 1980; Goodman et al., 1982;
Karsch et al., 1984).
Thus this pineal hormone appears to be essential for promoting continuity of estrous cycles once initiated.
In addition, melatonin has been found to suppress the secretion of prolactin in a number of species (Clark,
1980; Glass and Lynch, 1983).
This is consistent with data demonstrating that secretion of prolactin is maximal during spring and summer anestrus when ewes are exposed to long hours of daylight.
Thus, the patterns of secretion of these two hormones are inversely related.
With the exception of its effect on mammary gland function, the role(s) of prolactin in the ewe is not known.
During seasons of maximal secretion of melatonin and prolactin these hormones may act on distant target organs such as the uterus.
Evidence has been presented
56
that melatonin can
alter the
uterine estrogen receptor
concentrations in the hamster (Danforth et al., 1983) and
human breast cancer cells
(Danforth and Lippman, 1981).
However, these conclusions remain equivocal.
This experiment was conducted to determine whether concentrations of estrogen receptor in control and estradiolstimulated uteri of ovariectomized ewes differ between fall and summer.
A detected difference in this uterine
characteristic would suggest the possibility that
either melatonin or prolactin was acting either directly or indirectly to alter uterine function.
MATERIALS AND METHODS
Animals
Ten mature Polypay ewes were checked
twice daily for
estrus to ensure all ewes exhibited an estrous cycle of normal duration.
Ewes were then bilaterally ovariectomized
in
October and allowed 4-6 weeks for recovery.
At the height of the winter solstice, a 10 ml blood sample was collected by jugular venipuncture from each of five ewes for analyses of serum concentrations of estrogen and progesterone. These five ewes were then anesthetized and a midventral laparotomy was performed.
Approximately one-third to one-half of a uterine horn was removed and placed on ice.
The contralateral uterine horn was ligated at the external bifurcation and estradiol-
57
1713 (10 gg/3ml of physiological saline) was injected into the lumen of the contralateral horn to stimulate synthesis of estrogen receptors.
This dose of estradiol -178 was chosen because results of a preliminary experiment indicated lower doses of this steroid to be nonstimulatory.
It was assumed
that a dose of 10 leg would not, or at least be minimally
effective, in stimulating release of prolactin.
After 48 h ewes were relaparotomized and a section of the stimulated uterine horn was removed and placed on ice.
Endometrium (50 mg) was assayed within 90 min of removal for concentrations of estrogen receptors using the estrogen nuclear and cytosolic exchange methods.
The above procedure was repeated during the height of the summer solstice on the remaining ewes (n=5).
Comparisons were made between the concentration of estrogen receptors found in the endometrium of ewes during the winter and summer solstices,
as well as between stimulated and
nonstimulated endometrium.
Nuclear and Cytosolic Estrogen Receptor Assay
The assay of nuclear and cytosolic estrogen receptors was performed as described in experiment 1.
DNA Determination
Deoxyribonucleic acid concentration in the endometrial tissue was measured as described in experiment 1.
58
Serum Progesterone and Estradiol Radioimmunoassay
Systemic concentrations of progesterone and estradiol-
1713 were measured as described in experiment 1.
Statistical Analysis
Data on endometrial nuclear and cytosolic concentrations were analyzed by analysis of variance for an experiment of 2 x 2 factorial design.
The factors or main effects were season
(winter and summer) and exogenous estradiol (0 and 10 Ag).
RESULTS
Changes in endometrial nuclear and cytosolic concentrations of estrogen receptor before and after estradiol treatment at the winter and summer solstice are depicted in figure 3.
Nuclear concentrations of estrogen receptor in the
endometrium of chronically ovariectomized ewes prior
to exogenous estradiol stimulation during the time of the winter
solstice were not different
(p>.05)
from those prior to
estradiol stimulation during the summer solstice (0.46 ± 0.10
vs 0.46
± 0.07
fmol/Ag DNA, respectively).
Similarly, cytosolic concentrations of estrogen receptor in endometrium of ovariectomized ewes prior to exogenous estradiol
stimulation were not different at the winter and summer
solstice (2.64
± 1.45
vs 3.27
± 1.08
fmol/Ag DNA, respectively).
59
However, at both the winter and summer solstice, treatment of ewes with estradiol resulted in a significant increase in endometrial concentrations of nuclear and cytosolic estrogen receptors compared with concentrations present prior to treatment (p<.05).
Nuclear concentrations of estrogen receptor in endometrium of ovariectomized ewes 48 h after estradiol stimulation were 0.804 ± 0.20 fmol/Ag DNA during the winter solstice and 1.18 ± 0.46 fmol/Ag DNA during the summer solstice.
These mean nuclear concentrations of estrogen receptor were not significantly different.
Similarly, there was no significant difference detected in endometrial cytosolic concentrations of estrogen receptor of estradiol-treated ewes at the winter and summer solstice (6.09
± 2.22 fmol/Ag DNA vs 8.29 ± 1.17 fmol/Ag DNA; p>.05).
Serum concentrations of estradiol prior to treatment of ewes with estradiol during the winter solstice and summer solstice did not differ significantly (16.55 ± 4.05 vs 16.00
± 3.00 pg/ml of serum, respectively; p>.05).
Similiarly, serum levels of estradiol 48
h after administration of estradiol did not differ significantly among ewes at the winter and summer solstice (18.75 ± 2.40 vs 18.65 ± 1.65
pg/ml, respectively).
Serum levels of progesterone were basal (<0.10 ng/ml) confirming successful and complete ovariectomy of ewes.
ill WINTER
SUMMER
-E2 NUC -E2 CYT +E2 NUC +E2 CYT
Fig. 3.
Specifically bound Offlestradiol-
178 to nuclear and cytosolic estrogen receptors (mean ±
SE) in nonstimulated and exogenous estradiol-stimulated endometria of ewes during the winter and summer solstice.
Stimulated vs nonstimulated endometrium; nuclear (p<.05); cytosolic (p<.01).
60
61
DISCUSSION
Unlike the rabbit uterus, which becomes refractory to ovarian steroids when the interval between ovariectomy and hormone replacement is prolonged (Daniel, 1984), results of this experiment demonstrate that the uterus of the ewe is
responsive to exogenous steroids as long as
9
mo after
ovariectomy.
This is reflected by the fact that injection of
10 Ag of estradio1-1713 directly into the uterine lumen at 3 and 9 mo after ovariectomy induced increases in endometrial estrogen receptors compared with concentrations in control tissue.
No differences in concentrations of estrogen receptor were observed between either the estrogen-treated or control uterine horns of ewes during winter and summer.
These data
indicate that there is no nongonadal seasonal effect on
estrogen receptor concentrations in ovariectomized ewes. From these data, it does not appear that either prolactin (during summer anestrus), or melatonin
(during the fall breeding
season) are able to directly or indirectly alter the concentration of uterine estrogen receptors in ovariectomized ewes.
This observation is in contrast to those reported by
Danforth et al.
(1983) who found that melatonin was able to induce an increase in estrogen receptor concentrations in immature hamster uteri in vivo and in vitro.
Direct in vitro
62 regulation of estrogen receptor activity by melatonin has also been shown to occur in human breast cancer cells (Danforth and
Lippman, 1981).
Chilton and Daniel (1987) demonstrated that prolactin treatment of ovariectomized rabbits resulted in an increase in uterine concentrations of estrogen and
progesterone receptors similar to
those detected in the sexually receptive intact rabbit.
Serum concentrations of estradiol both prior to and after estradiol treatment were not different from one another indicating that treatment with estradiol did not increase systemic concentrations of estradiol and therefore did not likely provoke a release of prolactin.
Serum concentrations of progesterone detected in the ovariectomized ewes undoubtedly reflect steroid secreted from the adrenal cortex.
Because there was no significant difference in serum progesterone concentrations among ewes within season
(0 vs 10 Ag estradiol) or between seasons
(winter vs summer) it is unlikely that any slight variation in progesterone secretion could have accounted for failure to detect differences in endometrial estrogen receptor concentrations.
These data indicate that the mechanism by which seasonality regulates the breeding season is via an indirect route through the ovary.
The secretion of estrogen from the
ovary acts on the
uterine endometrium to stimulate the
synthesis of estrogen and progesterone receptors.
It appears
that melatonin secreted in greater quantities during the
63
breeding season of the ewe is acting at the
level of the hypothalamus to regulate release of GnRH and subsequently pituitary LH.
Likewise, increased daily secretion of
prolactin during summer anestrus does not appear to act
directly on the uterus to influence synthesis or concentrations of estrogen receptors and thereby promote this reproductive state.
Collectively, these data indicate that neither prolactin nor melatonin act directly on the uterine endometrium to cause fluctuations in the synthesis of estrogen receptors in the ovariectomized ewe.
64
BIBLIOGRAPHY
Abdelgadir, S.E., Swanson, L.V., Oldfield, J.E. and Stormshak,
F.
1987. Prostaglandin Fla- induced release of oxytocin from bovine corpora lutea in vitro. Biol. Reprod. 37:550.
Advis, J., Richards, J.S.
and Ojeda, S.R.
1981.
Hyperprolactinemia-induced precocious puberty: Studies on the mechanism(s) by which prolactin enhances ovarian progesterone responsiveness to gonadotropins in prepubertal rats. Endocrinology 108:1333.
Aitken, R.J. 1979. The hormonal control of implantation. In:
Maternal Recognition of Pregnancy. Ciba Found. Symp., No.
64. Excerpta Medica, Amsterdam. p.53.
Aitken, R.J., Bowman, P. and Gauld, I.
1977. The effect of synchronous and asynchronous egg transfer on fetal weight
in mice selected for large and small body size.
J.
Embryol. Exp. Morphol. 37:59.
Anthony, R.V., Helmer, S.D., Sharif, S.F., Roberts, R.M.,
Hansen, P.J., Thatcher, W.W.
and Bazer, F.W.
1988.
Synthesis and processing of ovine trophoblast protein-1 and bovine trophoblast protein-1, conceptus secretory proteins involved in the maternal recognition of pregnancy. Endocrinology 123:1274.
Astwood, E.B. 1938. A six hour assay for the quantitative determination of estrogen. Endocrinology 23:25.
Auletta, F.J. and Flint, A.P.F. 1988. Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation luteolysis. Endocrine Rev. 9:88.
to the time of
Avvakumov, G.V., Survilo, L.I., Strel'chenok, O.A.
1986.
Interaction of serum sex steroid-binding globulin with cell membranes of human decidual tissue. Biochem. Acad.
Sci. U.S.S.R. 50:983.
Baird, D.T. 1977. Evidence in vivo for the two-cell hypothesis of oestrogen synthesis by the sheep Graafian follicle.
J. Reprod. Fertil. 50:183.
Baird, D.T.
1978.
Local utero-ovarian relationships.
In:
Crighton, D.B., Haynes, G.R., Foxcroft, G.R. and Lamming,
G.E. (Eds.) Control of Ovulation. Butterworths, London.
pp. 217-232.
65
Baird, D.T., Land, R.B.,
Sacaramuzzi, R.J. and Wheeler, A.G.
1976. Endocrine changes associated with luteal regression in the ewe: The secretion of ovarian oestradiol, progesterone, androstenedione and uterine prostaglandin
Fez throughout the oestrous cycle. J. Endocrinol. 69:275.
Barley, D.A., Butcher, R.L.
and Inskeep, E.K.
1966. Local nature of utero-ovarian relationships pseudopregnant rat. Endocrinology.
79:119.
in the
Bazer, F.W. and First, N.L.
1983. Pregnancy and parturition.
J. Anim. Sci. 57(Supp1.2):425.
Bazer, F.W., Geisert, R.D.,
Thatcher, W.W. and Roberts, R.M.
1982. The establishment and maintenance of pregnancy. In:
Cole, D.S.A. and Foxcroft, G.R.
(Eds.) Control of Pig
Reproduction. Butterworth Scientific,
London.
Berthois, Y., Pourreau-Schneider, N.,
H. and Tubiana, N. 1986.
Gandilhon, P., Mittre,
Estradiol membrane binding sites on human breast cancer cell lines. Use of a fluorescent estradiol conjugate to demonstrate plasma membrane binding systems. J. Steroid Biochem. 25:963.
Bex,
F., Bartke, A., Goldman, B.D.
and Dalterio, S.
1978.
Prolactin, growth hormone, luteinizing hormone receptors and seasonal changes in testicular activities in the golden hamster. Endocrinology 103:2069.
Bindon, B.M. 1971. The role of progesterone in implantation in the sheep. Aust. J. Biol.
Sci. 24:149.
Bittman, E.L., Kaynard, A.H., Olster,
Yellon,
S.M. and Karsch, F.J.
D.H., Robinson, J.E.,
1985. Pineal melatonin mediates photoperiodic control of pulsatile luteinizing hormone secretion in the ewe. Neuroendocrinology 40:409.
Bjersing, L. 1967. On the morphology and endocrine function
of granulosa cells in ovarian follicles and corpora
lutea.
Acta Endocrinol. 56(Suppl. 125).
Boyar, R.M., Finkelstein,
J.W., Kapen, S.
and Hellman, L.
1975. Twenty-four hour prolactin (PRL) secretory patterns during pregnancy. J. Clin.
Endocrinol. Metab. 40:1117.
Brann, D.W., Rao, I.M. and Mahesh, V.B. 1988. Antagonism of estrogen-induced prolactin release by progesterone. Biol.
Reprod. 39:1067.
Burton, K. 1956. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem. J. 62:315.
66
Butcher, R.L.
1977. Changes in gonadotropins and steroids associated with unilateral ovariectomy in the rat.
Endocrinology 101:830.
Buttle, H.L. 1974. Seasonal variation of prolactin in plasma of male goats. J. Reprod. Fertil. 37:95.
Caffrey, J.L., Fletcher, P.W., Diekman, M.A., O'Callaghan,
P.L. and Niswender, G.D.
1979. The activity of ovine
luteal cholesterol esterase during several experimental conditions. Biol. Reprod. 21:601.
Caron, M.G., Goldstein, S., Savard, K. and Marsh, J.M. 1975.
Protein kinase stimulation of a reconstituted cholesterol side chain cleavage enzyme system in the bovine corpus luteum. J. Biol. Chem. 250:5137.
Carson, R.S., Findlay, J.K., Burger, H.G. and Trounson, A.O.
1979.
Gonadotropin receptors of the ovine ovarian follicle during follicular growth and atresia.
Biol.
Reprod. 21:75.
Casida, L.E. and Warwick, E.J.
1945. The necessity of the corpus luteum for maintenance of pregnancy in the ewe.
J. Anim. Sci. 4:34.
Chepenik, K.P.
and Smith, J.B.
1980.
Synthesis of prostaglandins by mouse embryos. Int. Res. Commun. Syst.
Med. Sci. 8:783.
Chilton, B.S. and Daniel, Jr., J.C. 1987. Differences in the rabbit uterine response to progesterone as influenced by growth hormone or prolactin. J. Reprod. Fertil. 79:581.
Clark, J.H. and Markaverich, B.M. 1988. Actions of ovarian steroid hormones. In: Knobil, E., Neill, J.D., Ewing,
L.L., Greenwald, G.S., Markert, C.L.
and Pfaff, D.W.
(Eds.) The Physiology of Reproduction. Raven Press, New
York. pp. 675-726.
Clark, J.R. 1980. Physiological problems of seasonal breeding in eutherian animals. Oxford Rev. Reprod. Biol. 2:244.
Cox, R.I., Mattner, P.E. and Thorburn, G.D. 1971. Changes in ovarian secretion of oestradiol -178 around oestrus in the sheep. J. Endocrinol. 49:345.
Cumming, I.A., Baxter, R. and Lawson, R.A.S. 1974. Steroid hormone requirement for the pregnancy in sheep
: maintenance of early
A study using ovariectomized,
adrenalectomized ewes. J. Reprod. Fertil. 40:443.
67
Dalton, D.L.
and Knight, J.W.
1983.
Effects of exogenous progesterone and estrone on conceptus development in swine. J. Anim. Sci. 56:1354.
Danforth, Jr., D.N., Tamarkin, L., Do, R. and Lippman, M.E.
1983. Melatonin-induced increase in cytoplasmic estrogen receptor activity in hamster uteri. Endocrinology 113:81.
Danforth, Jr., D.N. and Lippman, M.E. 1981.
Melatonin-induced increase in estrogen receptor concentration in MCF-7 human breast cancer cells.
Abstr.
63rd Annu.
Mtg.
Endocrine Soc. p. 247, Abstr. 660.
Daniel, J.C., Jetton, A.E. and Chilton, B.S. 1984.
Prolactin as a factor in the uterine response to progesterone in rabbits. J. Reprod. Fertil. 72:443.
DeHertogh, R., Ekka, E. and Vanderheyden, I. 1986.
Estrogen dependent induction of low affinity binding sites in the nuclear fraction of rat uterus. J. Steroid Biochem.
24:1171.
Denamur, R., Martinet, J. and Short, R.V.
1973. Pituitary control of the ovine corpus luteum. J. Reprod.Fertil.
32:207.
Djiane, J.
and Durand, P.
1977.
Prolactin-progesterone antagonism in self regulation of prolactin receptors in the mammary gland. Nature 266:641.
Donaldson, L.E., Hansel, W.
and van Vleck,
L.D.
1965.
Histological study of bovine corpora lutea.
J. Dairy
Sci. 48:331.
Dorrington, J.H. and Armstrong, D.T. 1979. Effects of FSH on gonadal function. Rec. Prog. Horm. Res. 35:301.
Dorrington, J. and Gore-Langton, R.E. 1981. Prolactin inhibits oestrogen synthesis in the ovary. Nature 290:600.
du Mesnil du Buisson, F.
1961. Regression unilaterale des corps jaunes apres hysterectomie partielle chez la truie.
Ann. Biol. Anim. Biochem. Biophys. 1:105.
Ekka, E., Vanderheyden, I., Glorieux, B. and DeHertogh, R.
1987. Estradiol-induced progesterone receptor synthesis in normal and diabetic ovariectomized rat uterus.
J.
Steroid Biochem. 28:61.
Eley, R.M., Thatcher, W.W. and Bazer, F.W. 1979. Hormonal and physical changes associated with bovine conceptus development. J. Reprod. Fertil. 55:181.
68
Ellinwood, W.E. 1978. Maternal recognition of pregnancy in the ewe and the rabbit. Ph.D
Dissertation. Colorado State
Univ., Fort Collins.
Espey, L.L. 1980. Ovulation as an inflammatory reaction--a hypothesis. Biol. Reprod. 22:73.
Evans, G., Dobias, M., King, G.J.
and Armstrong, D.T. 1981.
Estrogen, androgen and progesterone biosynthesis by theca and granulosa of preovulatory follicles in the pig. Biol.
Reprod. 25:673.
Falck, B. 1959. Site of production of ovary as studied by oestrogen in the rat microimplants. Acta Physiol. Scand.
47:94.
Farin, C.E., Imakawa, K., Hansen,
Murphy, C.N., Farin, P.W.
T.R., McDonnell, J.J., and Roberts, R.M.
1990.
Expression of trophoblastic interferon genes in sheep and cattle. Biol. Reprod. 43:210.
Fincher, K.B., Bazer, F.W., Hansen, P.J.,
Thatcher, W.W. and
Roberts, R.M. 1986. Ovine conceptus secretory proteins suppress induction of uterine prostaglandin Fla release by oestradiol and oxytocin. J. Reprod.
Fertil. 76:425.
Fischer, H.E., Bazer, F.W. and Fields, M.J.
1985. Steroid metabolism by endometrial and conceptus tissues during early pregnancy and pseudopregnancy in gilts. J. Reprod.
Fertil. 75:69.
Fischer, T.V. 1965. Local uterine inhibition of the corpus luteum in the guinea pig. Anat. Rec.
151:350.
Flint, A.P.F. and Sheldrick, E.L. 1986.
Ovarian oxytocin and the maternal recognition of pregnancy. J. Reprod. Fertil.
76:831.
Ford, S.P. 1982. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of the ewe, sow and cow. J. Anim.
Sci. 55(Suppl. 2):32.
Ford, S.P., Weems, C.W., Pitts, R.E.,
R.L. and Inskeep, E.K. 1975.
Pexton, J.E., Butcher,
Effects of estradiol -178 and
progesterone on prostaglandin F
in sheep uteri and
uterine venous plasma. J. Anim.
Sci. 41:1407.
Fortune, J.E. and Armstrong, D.T.
1978. Hormonal control of
178-estradiol biosynthesis in proestrous rat follicles:
Estradiol production by isolated theca versus granulosa.
Endocrinology 102:227.
69
Foster, D.L., Lemons, J.A., Jaffe, R.B. and Niswender, G.D.
1975.
Sequential patterns of circulating luteinizing hormone and follicle-stimulating hormone in female sheep
from early postnatal life through the first estrous
cycle. Endocrinology 97:985.
Gadsby, J.E., Heap, R.B. and Burton, R.D.
1980. Oestrogen production by blastocyst and early embryonic tissue of various species. J. Reprod. Fertil. 60:409.
Gardner, M.L., First, N.L. and Casida, L.E. 1963. Effect of exogenous estrogen on corpus luteum maintenance in gilts.
J. Anim. Sci. 22:132.
George, F.W. and Wilson, J.D. 1978. Estrogen formation in the early rabbit embryo. Science 199:200.
Ginther, O.J. 1979. Reproductive Biology of the Mare: Basic and Applied Aspects. McNaughton and Gunn, Inc. Ann Arbor,
MI.
Ginther, O.J. and First, N.L. 1971. Maintenance of the corpus luteum in hysterectomized mares.
Am.
J.
Vet.
Res.
32:1687.
Glass, J.D. and Lynch, G.R. 1983. Melatonin: Identification of sites of antigonadal action in mouse brain. Science.
214:821.
Glencross, R.G., Munro, I.B., Senior, B.E. and Pope, G.S.
1973.
Concentrations of estradio1-1713, oestrone and progesterone in jugular venous plasma of cows during the oestrous cycle and in early pregnancy. Acta Endocrinol.
73:374.
Goding, J.R. 1974. The demonstration that PGF2a is the uterine luteolysin in the ewe. J. Reprod. Fertil. 38:261.
Godkin, J.D., Bazer, F.W.
and Roberts, R.M.
1984a. Ovine trophoblast protein-1,
an early secreted blastocyst
protein, binds specifically to uterine endometrium and affects protein synthesis. Endocrinology 114:120.
Godkin, J.D., Bazer, F.W., Thatcher, W.W. and Roberts, R.M.
1984b.
Proteins released by cultured day 15-16 conceptuses prolong luteal maintenance when introduced into the uterine lumen of cyclic ewes. J. Reprod. Fertil.
71:57.
70
Goodman, R.L, Bittman, E.L.,
Foster, D.L. and Karsch, F.J.
1982. Alterations in the control of luteinizing hormonepulse
frequency underlie the
seasonal variation in estradiol negative feedback in the ewe.
Biol. Reprod.
27:580.
Gorski, J., Toft, D., Shyamala, G.,
1968. Hormone receptors:
Smith, D. and Notides, A.
Studies on the interaction of estrogen with the uterus. Rec.
Prog. Horm. Res. 24:45.
Greene, G.L.
structures and Press, M.F.
1986.
1.
Steroid receptor
(including monoclonal antibodies methods of determination). J.
and new
Steroid Biochem. 24:1.
Greenwald, G.S.
and Rothchild, I.
1968.
Formation and maintenance of corpora lutea in laboratory animals. J.
Anim. Sci. 27(Suppl. 1):139.
Greiss, Jr., F.C., and Anderson, S.G.
1970. Effect of ovarian hormones on the uterine vascular bed. Am.
J. Obstet.
Gynecol. 107:829.
Grota, L.J.
and Eik-Nes, K.B.
1967.
concentrations during pregnancy and
J. Reprod. Fertil. 13:83.
Plasma progesterone lactation in the rat.
Gwinner, E. and Dorka, V. 1976.
Endogenous control of annual reproductive rhythms in birds.
In: Proc.
16th Int.
Ornithol. Cong. Austr. Acad. of Sci., Canberra. pp.223.
Hafs, H.D. and Armstrong, D.T.
1968. Corpus luteum growth and progesterone synthesis during the bovine estrous cycle.
J. Anim. Sci. 27:134.
Halkerston, I.D.K., Eichhorn, J., and Hechter, 0.
1960.
Feinstein, M., Scully, E.
Early permeability effects of
estradiol on castrate rat uterus. Proc.
Soc. Exp. Biol.
Med. 103:796.
Hansel, W., Concannon, P.W.
and Lukaszewska, J.H.
1973.
Corpora lutea of the large
domestic animals.
Biol.
Reprod. 8:222.
Hansel, W. and Convey, E.M. 1983.
Physiology of the estrous cycle. J. Anim. Sci. 57(Suppl.
2):404.
Harper, M.J.K., Norris, C.J.
Prostaglandin
release by
and Rajkumar, V.
1983.
zygotes and pregnant rabbits. Biol. Reprod.
28:350.
endometria of
71
Hauger, R.L., Karsch, F.J.
1977. A new
concept for control of the estrous cycle of the ewe based on the temporal and Foster, relationships
D.L.
between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology 101:807.
Hisaw, Jr., F.L. 1959. Comparative effectiveness of estrogens on fluid imbibition
and growth of the rat's uterus.
Endocrinology 64:276.
Hixon, J.E. and Clegg, M.T. 1969.
Influence of the pituitary on ovarian progesterone output in the ewe: Effects of hypophysectomy and gonadotropic hormones.
Endocrinology
84:828.
Holt, J.A., Richards, J.S., Midgley, Jr.,
A.R. and Reichert
Jr., L.E. 1976. Effect of prolactin on LH receptor in rat luteal cells. Endocrinology. 98:1005.
Hotchkiss, J., Atkinson, L.E. and Knobil, E.
1971. Time course of serum estrogen and luteinizing hormone concentrations during the menstrual cycle of
(LH) the rhesus monkey. Endocrinology 89:177.
Hsueh, A.J.W., Peck, E.J. and Clark,
J.H. 1976. Control of uterine estrogen receptor levels by progesterone.
Endocrinology 98:438.
Huhtaniemi, I.T.
and Catt, K.J.
1981.
Induction and maintenance of gonadotropin and lactogen receptors in hypoprolactinemic rats. Endocrinology 109:483.
Hyland, J.H., Manns, J.G.
and Humphery, W. D.
Prostaglandin production by ovine embryos and
1982.
endometrium in vitro. J. Reprod. Fertil. 65:299.
Ichii, S., Forchielli, E. and Dorfman, R.I.
1963. In vitro effect of gonadotropins on the soluble cholesterol side chain cleaving enzyme system of bovine corpus luteum.
Steroids 2:631.
Inskeep, E.K.
and Butcher, R.L.
1966.
Local component of
utero-ovarian relationships in the ewe. J.
Anim. Sci.
25:1164.
Ireland, J.J.
1987.
Control of follicular growth development. J. Reprod. Fertil. Suppl. 34:39.
and
72
Jensen, E.V., Suzuki, T., Kawashima, T., Stumpf, W.E.,
Jungblut, P.W.
and DeSombre, E.R.
1968.
A two-step mechanism for the interaction of estradiol with rat
uterus. Biochemistry 59:632.
Kaltenbach, C.C., Graber, J.W., Niswender, G.D. and Nalbandov,
A.V. 1968. Effect of hypophysectomy on the formation and maintenance of corpora lutea in the ewe. Endocrinology
82:753.
Karsch, F.J. 1987. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Ann. Rev. Physiol. 49:365.
Karsch, F.J., Bittman, E.L., Foster, D.L., Goodman, R.L.,
Legan, S.J. and Robinson, J.E. 1984. Neuroendocrine basis of seasonal reproduction. Rec. Prog. Horm. Res. 40:185.
Karsch, F.J., Cook, B., Ellicott, A.R., Foster, D.L., Jackson,
G.L.
and Nalbandov, A.V.
1971.
Failure of infused prolactin to prolong the life span of the corpus luteum of the ewe. Endocrinology 89:272.
Karsch, F.J., Foster, D.L., Legan, S.J., Ryan, K.D. and Peter,
G.K. 1979. Control of the preovulatory endocrine events in the ewe: Interrelationship of estradiol, progesterone and luteinizing hormone. Endocrinology 105:421.
Karsch, F.J., Legan, S.J., Ryan, K.D. and Foster, D.L. 1980.
Importance of estradiol and progesterone in regulating
LH secretion and estrous behavior during the sheep
estrous cycle. Biol. Reprod. 23:404.
Kennaway, D.J., Dunstan, E.A., Gilmore, T.A. and Seamark, R.F.
1984. Effects of pinealectomy, oestradiol and melatonin on plasma prolactin and LH secretion in ovariectomized sheep. J. Endocrinol. 102:199.
King, W.J.
and Greene, G.L.
1984.
Monoclonal antibodies
localize oestrogen receptor in the nuclei of target
cells. Nature 307:745.
Klopper, A. 1983. Specific pregnancy proteins. In: Martin, L.
and James, V.H.T. (Eds.) The Endocrinology of Pregnancy and Parturition. Academic Press, New York. p. 127.
Knickerbocker, J.J., Thatcher, W.W., Bazer, F.W., Drost, M.,
Barron, D.H., Fincher, K.B.
and Roberts, R.M.
1986.
Proteins secreted by day 16 to 18 bovine conceptuses
extend corpus luteum function in cows. J. Reprod. Fertil.
77:381.
73
Knobil, E.
1973. On the regulation of the
luteum. Biol. Reprod. 8:246.
primate corpus
Koligian, K.B.
and Stormshak, F.
cytoplasmic estrogen receptors
1977a.
during the estrous cycle. Endocrinology
Nuclear and
in ovine endometrium
101:524.
Koligian, K.B.
and Stormshak, F.
1977b.
Progesterone inhibition of estrogen receptor replenishment in ovine endometrium. Biol. Reprod. 17:412.
Lemon, M. and Loir, M. 1977. Steroid release in vitro by two luteal cell types in the corpus luteum of the pregnant sow. J. Endocrinol.
72:351.
Leung, P.C.K.
and Armstrong, D.T.
1980.
Interactions of steroids and gonadotropins in the control of steroidogenesis
in the ovarian
follicle.
Ann.
Rev.
Physiol. 42:71.
Ling, W.Y. and Marsh, J.M. 1977.
Reevaluation of the role of cyclic adenosine 3',5'-monophosphate and protein kinase
in the stimulation of
steroidogenesis by luteinizing hormone in bovine corpus luteum slices. Endocrinology
100:1571.
Liu, Y.-X. and Hsueh, A.J.W. 1986. Synergism between granulosa and theca-interstitial cells in estrogen biosynthesis by gonadotropin-treated rat ovaries: Studies on the two-cell two-gonadotropin hypothesis using steroid antisera. Biol.
Reprod. 35:27.
Mais, V., Kazer, R.R., Cetel, N.S.,
Rivier, J., Vale, W. and
Yen, S.S.C. 1986. The dependency of folliculogenesis and corpus luteum function on pulsatile secretion in cycling women using a gonadotropin gonadotropin-releasing
hormone antagonist as a probe.
J.
Clin.
Endocrinol.
Metab. 62:1250.
Marcus, G.J. 1981. Prostaglandin formation by the sheep embryo and endometrium as an indication of maternal recognition of pregnancy. Biol. Reprod. 25:56.
Marsh, J.M. 1975. The role of
Biol. Reprod. 14:30.
cyclic AMP in steroidogenesis.
Martal, J.M., LaCroix, M.C., Loudis, C., Saunier, M.
Winterberger-Torres, S.
1979.
Trophoblastin, and an antiluteolytic protein present in early pregnant sheep.
J. Reprod. Fertil. 56:63.
74
Martin, L., Hallowes, R.C., Finn, C.A. and West, D.G. 1973.
Involvement of the uterine blood vessels in the
refractory state of the uterine stroma which follows
oestrogen stimulation in progesterone treated mice. J.
Endocrinol. 56:309.
Maywood, E.S., Buttery, R.C., Vance, G.H.S., Herbert, J.
and
Hastings, M.H. 1990. Gonadal responses of the male Syrian hamster to programmed infusions of melatonin are sensitive to signal duration and frequency but not to
signal phase nor to
lesions of the suprachiasmatic nuclei. Biol. Reprod. 43:174.
McCracken, J.A., Glew, M.E. and Scaramuzzi, R.J. 1970. Corpus luteum regression induced by prostaglandin F2 a. J.
Clin.
Endocrinol. Metab. 30:544.
Mercer, W.D., Edwards, D.P., Chamness, G.C. and McGuire, W.L.
1981. Failure of estradiol immunofluorescence in MCF-7 breast cancer cells to detect estrogen receptors. Cancer
Res. 41:4644.
Miller, B.G. and Moore, N.W. 1976. Effects of progesterone and oestradiol on RNA and protein metabolism in the genital tract and on survival of embryos in the ovariectomized ewe. Aust. J. Biol. Sci. 29:565.
Miller, M.A., Mullick, A., Greene, G.L. and Katzenellenbogen,
B.S.
1985. Characterization of the subunit nature of nuclear estrogen receptors by chemical cross linking and dense amino acid labeling. Endocrinology 117:515.
Miller, B.G., Murphy, L.
and Stone, G.M.
1977.
Hormone receptor levels and hormone, RNA and protein metabolism in the genital tract during the oestrous cycle of the ewe. J. Endocrinol. 73:91.
Miller, W.R. and Turner, C.W. 1963. Biosynthesis of steroids
from pregnenolone-16-3H by bovine ovarian
tissue in vitro. Steroids. 2:657.
Mitchell, M.D., Flint, A.P.F.
and Turnbull, A.C.
1975.
Stimulation by oxytocin of prostaglandin F levels in uterine venous effluent in pregnant and puerperal sheep.
Prostaglandins 9:47.
Moor, R.M. 1977. Sites of steroid production in ovine Graafian follicles in culture. J. Endocrinol. 73:143.
Moor, R.M. and Rowson, L.E.A. 1959.
Maintenance of pregnancy in ovariectomized ewes by means of progesterone. Nature
184:1410.
75
Moor, R.M. and Rowson, L.E.A. 1966a. Local maintenance of the corpus luteum
in sheep with embryos transferred to various isolated portions of the uterus.
J.
Reprod.
Fertil. 12:539.
Moor, R.M. and Rowson, L.E.A. 1966b. The corpus luteum of the sheep: Functional relationships between the embryo and the corpus luteum. J. Endocrinol. 34:233.
Moore, R.Y. and Klein, D.C. 1974. Visual pathways and the central neural control of a circadian rhythm in pineal serotonin N-acetyltransferase activity. Brain Res. 71:17.
Moudgal, N.R., Macdonald, G.J. and Greep, R.O. 1971.
Effect of hCG antiserum on ovulation and corpus luteum function in the monkey. J. Clin. Endocrinol. Metab. 32:579.
Mueller, G.C., Herranen, A.M. and Jervell, K.F. 1957.
Studies on the mechanism of action of estrogens. Rec. Prog. Horm.
Res. 14:95.
Muldoon, T.G. 1981. Interplay between estradiol and prolactin in the regulation of steroid hormone receptor levels, nature, and functionality in normal mouse mammary tissue.
Endocrinology 109:1339.
Nalbandov, A.V. 1971. Endocrine control of implantation. In:
Blandau, R.J.
(Ed.)
The Biology of the Blastocyst.
University of Chicago Press, Chicago. Chapter 22.
Neill, J.D.
1974.
Prolactin:
Endocrinology 4:469.
Its secretion and control.
Nicoll, C.S.
1974. Physiological actions of prolactin. In:
Greep, R.O.
and Astwood, E.B.
(Eds.) Handbook of
Physiology. Am. Physiol. Soc. Washington, D.C. pp. 253-
292.
Niswender, G.D., Moore, R.T., Akbar, A.M., Nett, T.M.
and
Diekman, M.A.
1975. Flow of blood to the ovaries of
cycling and pregnant ewes. Biol. Reprod. 13:38.
Niswender, G.D. and Nett, T.M. 1988. The corpus luteum and its control. In: Knobil, E., Neill, J.D. Ewing, L.L.,
Greenwald, G.S., Markert, C.L. and Pfaff, D.W. (Eds.) The
Physiology of Reproduction. Raven Press, New York. pp.
489-525.
Niswender, G.D., Reimers, T.J., Diekman, M.A. and Nett, T.M.
1976. Blood flow: A mediator of ovarian function. Biol.
Reprod. 14:64.
76
Niswender, G.D., Schwall, R.H., Fritz, T.A., Farin, C.E. and
Sawyer, H.R.
1985.
Regulation of luteal function in
domestic ruminants: New concepts. Rec. Prog. Horm. Res.
41:101.
Norman, A.W. and Litwack, G.L. 1987. Hormones. Academic Press,
San Diego. pp. 101-130.
Northey, D.L. and French, L.R. 1980. Effect of embryo removal and intrauterine infusion of embryonic homogenates on the lifespan of the bovine corpus luteum.
J.
Anim.
Sci.
50:298.
Pant, H.C., Hopkinson, C.R.N.
and Fitzpatrick, R.J.
1977.
Concentration of oestradiol, progesterone, luteinizing hormone and follicle stimulating hormone in the jugular
venous plasma of ewes during the oestrous
cycle.
J.
Endocrinol. 73:247.
Parikh, I., Ranjendran, K.G., Su, J.L., Lopez, T. and Sar, M.
1987. Are estrogen receptors cytoplasmic or nuclear?
Some immunocytochemical and biochemical studies.
J.
Steroid Biochem. 27:185.
Pavlik, E.J. and Coulson, P.B. 1976. Modulation of estrogen receptors in four different target tissues: Differential effects of estrogen vs progesterone. J. Steroid.
Biochem.
7:369.
Perry, J.S., Heap, R.B.
and Amoroso, E.C.
1973.
Steroid hormone production by pig blastocysts. Nature 245:45.
Peters, R.R.
hormone and Tucker, H.A.
1978.
Prolactin and growth responses to photoperiod in heifers.
Endocrinology 103:229.
Pope, W.F., Maurer, R.R. and Stormshak, F. 1982.
Survival of porcine embryos after asynchronous transfer. Proc. Soc.
Exp. Biol. Med. 171:179.
Posner, B.I., Kelly, P.A., Shiu, R.P.0 and Friesen, H.G.
1974.
Studies of insulin, growth hormone and prolactin binding; tissue distribution, special variation and characterization. Endocrinology 95:521.
Pratt, B.R., Butcher, R.L.
and Inskeep, E.K.
1977.
Antiluteolytic effect of the conceptus and of PGE2 in ewes. J. Anim. Sci. 46:784.
77
Reynolds, L.P., Magness, R.R. and Ford., S.P. 1982.
Uterine
blood flow
(UBF)
and uterine arterio-venous steroid
concentration on d 11, 13, and 15 of the estrous cycle and pregnancy in ewes. J. Anim. Sci. 55(Suppl. 1):382.
Riddick, D.H. 1985. Regulation and physiological relevance of non-pituitary prolactin. In: MacLeod, R.M., Thomer, M.O.
and Scapagrini, U.
(Eds.) Prolactin Basic and Clinical
Correlates. V. Liviana Press, Padova. pp. 463-473.
Roberts, K.D., Bandy, L.
and Leiberman, S.
1967.
The
conversion
of cholesterol-3H-sulfate-35S into pregnenolone-3H-sulfate-35S by sonicated bovine adrenal mitochondria. Biochem. Biophys. Res. Commun. 29:741.
Rodgers, R.J. and O'shea, J.D. 1982.
Purification, morphology and progesterone production and content of three cell types isolated from the corpus luteum of sheep. Aust.
J.
Biol. Sci. 35:441.
Rolland, R., Grusalus, G.L.
and Hammond, J.M.
1976.
Demonstration of specific binding of prolactin by porcine corpora lutea. Endocrinology 98:1083.
Rose, J., Oldfield, J.E. and Stormshak, F. 1986.
Changes in serum prolactin concentrations and ovarian prolactin
receptors during embryonic diapause
in mink.
Biol.
Reprod. 34:101.
Rothchild, I.
1981. The regulation of the mammalian corpus luteum. Rec. Prog. Horm. Res. 37:183.
Sabbah, M., Redeuilh, G.
and Baulieu, E.E.
1989.
Subunit composition of the estrogen receptor. Involvement of the hormone-binding domain in the dimeric state. J. Biol.
Chem. 264:2397.
Sar, M. and Meites, J. 1968. Effects of suckling on pituitary release of prolactin, GH and TSH in post-partum lactating rats. Neuroendocrinology 4:25.
Schallenberger, E., Schams, D., Bullermann, B. and Walters,
D.L. 1984. Pulsatile secretion of gonadotropins, ovarian steroid and ovarian oxytocin during prostaglandin induced regression of the corpus luteum in the cow. J. Reprod.
Fertil. 71:493.
Schams, D. and Karg, G. 1974. Prolactin release in cattle. J.
Reprod. Fertil. 39:463.
78
Schomberg, D.W., Coudert, S.P. and Short,
R.V. 1967. Effects of bovine luteinizing hormone and human chorionic gonadotropin on the bovine corpus luteum in vivo. J.
Reprod. Fertil. 14:227.
Schwartz, N.B. and Channing, C.P. 1977.
Evidence for ovarian
"inhibin" suppression of the secondary
rise in serum
follicle stimulating hormone levels in proestrous rats by injection of porcine follicular fluid. Proc. Natl.
Acad. Sci. U.S.A. 74:5721.
Selcer, K.W.
and Leavitt, W.W.
1988.
Progesterone downregulation of nuclear estrogen receptor: A fundamental mechanism in birds and mammals. Gen. Comp.
Endocrinol.
72:443.
Senior, B.E. 1975. Cytoplasmic oestradiol-binding sites and their relationship to oestradiol content in the endometrium of cattle. J. Reprod. Fertil. 44:501.
Sheldrick, E.L. and Flint, A.P.F.
1983. Regression of the corpora lutea in sheep in response to cloprostenol is not affected by loss of luteal oxytocin after hysterectomy.
J. Reprod. Fertil. 68:155.
Sheldrick, E.L., Mitchell, M.D.
and Flint, A.P.F.
1980.
Delayed luteal regression in ewes
immunized against oxytocin. J. Reprod. Fertil. 59:37.
Shemesh, M., Milaguir, F., Ayalon, N.
and Hansel, W.
1979.
Steroidogenesis and prostaglandin synthesis by cultured bovine blastocysts. J. Reprod. Fertil. 56:181.
Short, R.V. 1962. Steroids in the follicular fluid and the corpus luteum of the mare.
A two-cell type theory of ovarian steroid synthesis. J. Endocrinol.
24:59.
Short, R.V. 1969. Implantation and pregnancy.
maternal recognition of
In: Wolstenholme, G.E.W. and O'Conner,
(Eds.) Fetal Autonomy. Churchill, London. pp.
2-26.
M.
Shutt, D.A., Clark, A.H., Fraser, I.S., Goh, P., McMahon,
G.R., Saunders, D.M. and Shearman,
R.P. 1976. Changes in concentration of prostaglandin F and steroids in human corpora lutea in relation to growth of the corpus luteum and luteolysis. J. Endocrinol. 71:453.
Silvia, W.J., Fitz, T.A., Mayan, M.H.
and Niswender, G.D.
1984.
Cellular and molecular mechanisms
involved in
luteolysis and maternal recognition of pregnancy in the ewe. Anim. Reprod. Sci. 7:57.
79
Singh, M.M. and Booth, W.D.
1979. Origin of oestrogen in
preimplantation rabbit blastocysts. J. Steroid Biochem.
11:723.
Sirois, J. and Fortune, J.E. 1988. Ovarian follicular dynamics during the estrous cycle in heifers monitored by realtime ultrasonography. Biol. Reprod. 39:308.
Slayden, 0. 1990. Endocrine Regulation of in Mink.
Ph.D.
Uterine Physiology
Dissertation.
Oregon State Univ.,
Corvallis Oregon.
Smeaton, T.C. and Robertson, H.A. 1971. Studies on the growth and atresia of Graafian follicles in the ovary of the sheep. J. Reprod. Fertil. 25:243.
Stetson, M.H. and Watson-Whitmyre, M.
1976. Nucleus suprachiasmaticus: The biological clock in hamsters?
Science
191:197.
Stewart, H.J., McCann, S.H.E., Barker, P.J., Lee, K.E.,
Lamming, G.E. and Flint, A.P.F. 1987. Interferon sequence homology and receptor binding activity of ovine trophoblast antiluteolytic protein.
J.
Endocrinol.
115:13.
Stone, G.M., Murphy, L.
and Miller, B.G.
1978.
Hormone receptor levels and metabolic activity in the uterus of the ewe: Regulation by oestradiol and progesterone.
Aust.
J. Biol. Sci. 31:395.
Stormshak, F., Kelley, H.E., and Hawk, H.W. 1969. Suppression of ovine luteal function by 1713 - estradiol. J.
Anim. Sci.
29:476.
Swanson, L.V., Hafs, H.D.
and Morrow, D.A.
1972. Ovarian characteristics and serum LH, prolactin, progesterone and glucocorticoids from first estrus to breeding size in holstein heifers. J. Anim. Sci. 34:284.
Thorpe, P. and Herbert, J. 1976.
Studies on the duration of the breeding season and photorefractoriness in female ferrets pinealectomized or treated with melatonin. J.
Endocrinol. 70:255.
Tindal, J.S.
1969. Central pathways involved in prolactin secretion.
In: Reynolds, M.
and Folley, S.
(Eds.)
Lactogenesis: The Initiation of Milk Secretion at
Parturition. Univ. of Penn. Press, Philadelphia, PA. pp.
253-256.
80
Tsai-Morris, C.-H., Ghosh, M., Hirshfield, A.N., Wise, P.M.
and Brodie, A.M.H. 1983. Inhibition of ovarian aromatase by prolactin in vitro. Biol. Reprod. 29:342.
Tsang, B.K. and Ooi, T.C. 1982. Prostaglandin secretion by human endometrium in vitro.
Am.
J.
Obstet.
Gynecol.
142:626.
Turnbull, K.E., Braden, A.W.H. and Mattner, P.E. 1977. The pattern of follicular growth and atresia in the ovine ovary.
Aust. J. Biol. Sci. 30:229.
Uresley, J.
and Leymarie,
P.
1979.
Varying response to luteinizing hormone of two cell types isolated from
bovine corpus luteum. J. Endocrinol. 83:303.
Vekemans, M. and Robyn, C. 1975. The influence of exogenous
estrogen on the circadian periodicity of circulating
prolactin in women. J. Clin. Endocrinol. Metab. 40:886.
Walters, D.L., Schams, D., Bullermann, B. and Schallenberger,
E.
1983. Pulsatile secretion of gonadotropins, ovarian steroids and ovarian oxytocin during luteolysis in the cow. Biol. Reprod. 28(Suppl. 1):142.
Wang, C. and Chan, V. 1982. Divergent effects of prolactin on estrogen and progesterone production by granulosa cells of rat Graafian follicles. Endocrinology 110:1085.
Wang, C., Hsueh, A.J.W. and Erickson, G.F. 1980. Prolactin inhibition of estrogen production by cultured rat granulosa cells. Mol. Cell. Endocrinol. 20:135.
Webster, G.M. and Haresign, W. 1983. Seasonal changes in LH and prolactin concentrations in ewes of two-breeds. J.
Reprod. Fertil. 67:465.
Welshons, W.V., Lieberman, M.E. and Gorski, J. 1984. Nuclear localization of unoccupied oestrogen receptors. Nature
307:747.
West, N.B., Hess, D.L. and Brenner, R.M. 1986. Differential suppression of progesterone receptors by progesterone in the reproductive tract of female macaques. J. Steroid
Biochem. 25:497.
Westpahl, U. 1983. Corticosteroid-binding globulin. A review of some recent aspects. Mol. Cell. Biochem. 55:145.
81
Wilde, D.E., Culver, A.A., Morcom,
1976.
C.B. and Dukelow, W.R.
Effect of administration of progesterone and estrogen on litter size in
pigs.
J.
Reprod. Fertil.
48:209.
Williams, M.F.
1948. The vascular architecture
of the rat
uterus as influenced by estrogen and progesterone. Am.
J. Anat. 83:247.
Williams, G.H., Hammond, J.M., Weisz, J.
and Mortel, R. 1978.
Binding sites for lactogenic hormone in the rat uterus.
Biol. Reprod. 18:697.
Wu, J.T.
1985.
Metabolism of progesterone by hamster blastocysts and the ontogeny of progesterone metabolic capability. Biol. Reprod. 33:53.
Yamamoto, K.R. 1985. Steroid receptor regulated transcription of specific genes and gene networks.
Ann. Rev. Genet.
19:209.
Yen, S.C., Tsai, C.C., Naftolin, F.,
Ajabor, L.
1972.
Vanderberg, G.
and
Pulsatile patterns of gonadotropin
release in subjects with and without ovarian function.
J. Clin. Endocrinol. Metab. 34:671.
Zavy, M.T., Mayer, R., Vernon,
D.C.
1979.
M.W., Bazer, F.W. and Sharp,
An investigation of the
uterine luminal environment of nonpregnant and pregnant pony mares.
J.
Reprod. Fertil. Suppl. 27:403.
Zeleznik, A.J., Midgley, Jr., A.R. and Reichert, Jr., L.E.
1974. Granulosa cell maturation in the rat: Increased binding of human chorionic gonadotropin following treatment with follicle stimulating hormone in vitro.
Endocrinology 95:818.
Zelinski, M.B., Hirota, N.A., Keenan, E.J.
and Stormshak, F.
1980. Influence of exogenous estradiol -178 on endometrial progesterone and estrogen receptors phase of the ovine estrous cycle.
during the luteal
Biol. Reprod. 23:743.
Zelinski, M.B., Noel, P., Weber, D.W.
and Stormshak, F. 1982.
Characterization of cytoplasmic progesterone receptors in the bovine endometrium during proestrus and diestrus.
J. Anim. Sci. 55:376.