137 MICROWAVE SPECTRUM, CONFORMATION, DIPOLE MOMENT AND CENTRIFUGAL
advertisement

137
Journal of Molecular Structure, 15 (1973) 137-150
~ Elsevier Scientific Publishing Company, Amsterdam - Printed in The Netherlands
MICROWAVE SPECTRUM, CONFORMATION, DIPOLE MOMENT AND
CENTRIFUGAL DISTORTION OF GLYOXYLIC ACID
K.-M.
MARSTOKK
Department
AND HARALD
of Chemistry,
MØLLENDAL
The University
of Oslo, Blindern,
Oslo 3 (Norway)
(Received 5 July 1972)
ABSTRACT
Microwave spectra of CHO-COOH and CHO-COOD are reported. The
molecule has a planar equilibrium conformation with the two carbonyl groups
trans to each other. A weak five-member intramolecular hydrogen bond is formed
between the hydroxyl proton of the carboxyl group and the oxygen atom of the
carbonyl group thus stabilizing the trans planar form. Other conformations having
a statistical weight of l (ds and trans) are at least 1.3 kcal mol-1 less stable, and
rotamers with a statistical weight of 2 (e.g., gauche and skew) have at least l. 7 kcal
mol-l higher energy. Four vibrationally excited states of CHO-COOH have been
analyzed and relativeintensity measurementsyielded 167:t 12 cm-1 for the C-C
torsional mode and 288:t 26 cm -1 for the lowest in-plane bending mode. The
dipole moment was determined to be Pa = 1.85:tO.03 D, Pb = O.20:tO.1OD, and
Ptot = 1.86:tO.04 D. A seven-parameter centrifugal distortion analysis has been
carried out for the ground vibrational state of CHO-COOD and for the ground
and three vibrationally excited states of CHO-COOH.
INTRODUCTION
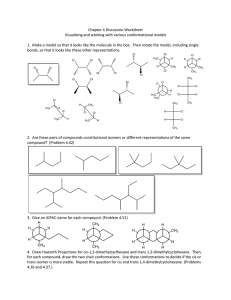
Several conformations are theoretically possible for free anhydrous glyoxylic
acid. In Fig. 1, a few of these are depicted. Very recently, Fleury and Tabacik1 have
shown by infrared and Raman spectroscopy that gaseous CHO-COOH exists in
a trans form, but they did not differentiate between trans 1 and trans 2 of Fig. I.
In the former case, a weak five-member intramolecular hydrogen bond may be
formed, whereas a four-member hydrogen bond may be forme d for trans 2. The
energy difference between these two trans conformations is probably not great,
e.g., the most stable form of the carboxyl group of free monomeric formic acid2. 3
138
H
o
,--<' 1-<'
TRANS 1
TRANS 2
'tb,H
H
45'H
SKEW
GAUCHE
~
o
C
C
H
O
H
CIS
Fig. 1. Five possible conformations of glyoxylic acid. Trans I, trans 2 and ds conformers are
planar, whereas skew and gauche are non-plan ar conformations reached by rotating the carbonyl
and the carboxyl groups relative to each other. The Newman projections show n are viewed alon g
the central C-C bond.
Trans I differs from trans 2 with respect to the position of the hydroxyl proton. For the
former rotamer a five-memberintramolecular hydrogen bond is formed, whereas a four-member
hydrogen bond is forrned for trans 2.
and acetic acid4 is similar to the one shown for trans 2, whereas the preferred form
of oxalic acid is similar to trans l. On the other hand, monofluoroacetic acid5
exists as cis and trans forms with respect to the C-C bond. In the former case, the
hydroxyl proton forms a hydrogen bond with the fluorine atom and the conformation of the carboxyl group is similar to trans l, whereas the configuration of the
carboxyl gro up is similar to trans 2 for the trans rotamer.
In addition to the conformational properties of the COOH group discussed
above, rotations around the C-C bond may produce distinct rotamers. For
molecules of the general type C101XY only the trans form has been found for
oxalic acid6. Glyoxal7 exists with the trans form 3.2 kcal mol-l more stable than
the cis form and oxalyl chloride8 (X = CI, y = CI) exists as trans and gauche
rotamers.
The present work was undertaken mainiy to study the conformational
behaviour of glyoxylic acid with particular regard to the influence of the intramolecular hydrogen bond on such molecular properties. It was found that
CHO-COOH exists mainly in the trans l configuration and that other rotamers
are present in concentrations not exceeding a total of 10 %.
139
EXPERIMENT
AL
Glyoxylic acid monohydrate was purchased from Schuchardt, Munich. The
monohydrate was placed over PzOs for several months until syrupy anhydrous
CHO-COOH formed. Crystallization of the free acid did not occur. CHO-COOD
was prepared by dissolving the monohydrate in DzO and drying for several months
over PzOs. The wave guide was seasoned with DzO before measurements were
made for the deuterated species.
The spectrometer was of the conventional 50 kHz Stark modulation type
and has been described briefly before9. Measurements were perforrned in the
12.4-18 GHz and the 22-36.3 GHz spectral regions. Recorder presentation of the
spectra was used and the major part of the upper spectral region was searched
extensively for the parent species. The molecule had an apparent vapour pressure
of 20 microns at room temperature at which the measurements were made. The
sample tubes were heated with a heat gun in order to rapidly achieve sufficient
vapour pressure. During this process the compound was seen to decompose slightly
forming formaidehyde as one of the decomposition products in agreement with
previous observationsl. The existence of the latter molecule was easily verified by
its strong and welI-characterized microwave spectrumlO.
METHOD OF CALCULATION
Dowlingll has shown that the first-order energy expression for a semirigid
planar asymmetric rotor may be written as
W = WJ,t(A', B', C')+tT~aaa<P:>
+tT~bbb<P:>
+t<ccc<P:>+
+*T~abb<P;P;
+ P;P;>.
(1)
The rotational constants A', B', and C' are related to the effective rotational
constants of a vibrational state v through
Av
Bv
Cv
= A ,-2 l h 4Tabab
4
= B ' -2 l h Tabab
4
= c , +4 3 h Tabab
(2)
with
1
A'
h4Tabab = !T~abb+ 4:
[( B'
Z
)
T~bbb+
B'
Z
()
A'
A'B'
<aaa-
Z
( )
C'Z
<cec J
.
(3)
The corrections to the rotational constants introduced by Tababare normalIy quite
small.
The computer programme used is a modification of MB07 described
previously9. This programme generates the energy matrices in the l' representation
140
(ref. 12) employing A', B', and C'. In the least-squares procedure the first-order
partial derivatives OWjOPiare needed. Pi are the seven parameters A', B', C',
'~aaa'!~bbb'<eec, and !~abb'The partial derivatives were computed in the following
manner. <P;) and <Pa4) were calculated by transferring K:' 1 and K~l'
respectively, from the rigid symmetric rotor basis to the rigid asymmetric rotor
basis by employing the eigenvectors obtained by diagonalizing the gI. and OI
matrices of King, Hainer and Cross12 using A', B', C' to calculate the asymmetry
parameter K. <pl) and <pc2)were calculated from
<p2) = [WJ,t(A', B', C')-C'J(J+1)-(A'-C')<P;)J
b
B'-C'
(4)
J(J+1) = <P;)+<P;)+<P~)
(5)
and
respectively.
The formulae given by COOk13 were used to obtain <Pb4), <P:), and
<P;P;+P;P;)
employing WJ,t(A', B', C'). The least-squares treatment of the
perturbation expression of eqn. (l) was found to yield standard deviations of the
fit (J = (I(Vicalc-Viobs)2jN)+ of about the same accuracy as the measurements
themselves. Convergence was normally achieved after 2-4 cycles. The calculations
are numerically very critica114 and were carried out on a CDC 6600 computer
with extended length arithmetics (about 29 digits). The results were in the case of
glyoxylic acid found to be numerically stable even for values of J exceeding 60.
MICROWAVE
SPECTRUM
AND ASSIGNMENT
OF THE GROUND
VIBRATIONAL
STATE
Preliminary rotational constants of several possible conformations of
glyoxylic acid were obtained by combining structural parameters transferred from
oxalic acid6 and glyoxa115 (see Table 1) and varying the appropriate dihedral
angles. Dipole moments and their components along the principal axes were
predicted for these rota mers by combining bond moments taken from ref. 16,
and by employing molecular orbital computations utilizing the semiempirical
CNDOj2 method 17 (complete neglect of differential overlap) known to give quite
reliable dipole moments in most cases18. The CNDOj2 method was also exploited
in an attempt to predict stable configurations of the acid, but, as expected, fallacious results were obtained for this molecule because of the presence of conjugation
(ref. 19) between the two carbonyl groups.
Glyoxylic acid is closely related to oxalic acid existing mainiy as trans, and
we therefore started searching for the trans conformations of the former molecule.
The strong a-type J = 2 --+3, 3 --+4, and 4 --+5 transitions were found close to
those predicted for the trans forrns. These lines have very clear Stark patterns and
141
TABLE I
PLAUSIBLE
GLYOXYLIC
STRUCTURAL
PARAMETERSa
AND OBSERVED AND PREDICTED
ROTATIONAL
CONSTANTS
OF
ACID
c-c
CO
C-O
C-H
O-H
1.548
1.208
1.339
1.114
1.056
Å
Å
Å
Å
Å
L CCOhYdroxy.
117.50
111.90
L CCOcarbonyl
LCOH
123.10
104.4°
LHCC
Rotationa/ constants (MHz)
---Ca/cu/ated
Observed
CHO-COOH
Ao
Bo
Co
10966.813
4605.988
3242.092
11075.11
4565.96
3233.06
CHO-COOD
Ao
Bo
Co
10422.262
4600.668
3190.305
10564.90
4554.03
3182.29
a
Not a derived structure. See text.
small centrifugal distortion perturbations. Preliminary rotational and centrifugal
distortion constants were obtained from the se transitions and used to predict the
a-type Q-branch transitions whose medium J transitions were subsequently found
dose to the predicted frequencies. Several of the low J Q-branch transitions had
resolvable Stark effect confirming their assignments. The assignment was quite
easily extended to high J values (up to J = 61) of the /1K- I = O, /1K1= + l
Q-branch series, because of the small centrifugal distortion perturbations of these
lines and also because of the relative ly simple nature of the spectrum. The spectrum
is presented in Table 2 and the rotational and centrifugal distortion constants
obtained by least squares fitting the measured lines to eqn. (1) is presented in
Table 4. As can be seen from the latter table, the rotational constants are determined very accurately, and these were used to predict low J b-type lines of comparatively high intensities. A search for several such lines were made, but none
were found owing to the small component of dipole moment along the b-axis
being 0.20:!:0.10 D thus producing insufficient intensities.
The observed inertial defect L1 = /e-/a-/b = 0.07578 UA2 is similar in
magnitude and sign to the values observed for completely planar molecules.
The 1east-squares procedure is only capable of determining three of the
centrifugal distortion constants, viz., 1:~aaa'
1:~bbb'
and 1:~abb'
whereas 1:;eee
is essentially
undetermined. This constant is defined to have a negative value2O, but the leastsquares treatment yield a positive and small constant with a standard deviation of
the same magnitude as <eec itself. Moreover, the four centrifugal distortion coefficients are strongly correlated as shown in Table 3.
142
TASLE 2
SPECTRAL DATA FOR CHO-COOH AND CHO-COOD
Ground vib.
state of CHO-COOH
Transition
1
1
2
2
2
3
3
3
3
3
3
3
4
4
4
5
6
7
8
9
11
12
13
14
15
16
17
18
19
20
21
22
24
27
29
30
31
32
34
39
40
41
44
47
48
51
54
58
61
"
1
1
O
2
2
1
O
3
3
2
2
1
1
O
O
O
1
1
2
2
3
3
4
4
4
5
5
5
6
6
6
7
7
8
9
9
9
10
10
12
12
12
13
14
14
15
16
17
18
ObserJied
freq.
(MHz)
1
O
2
1
O
3
3
1
O
2
1
2
4
4
4
5
6
7
7
8
9
10
10
11
12
12
13
14
14
15
16
16
18
20
21
22
23
23
25
28
29
30
32
34
35
37
39
42
44
2
2
3
3
3
4
4
4
4
4
4
4
5
5
4
5
6
7
8
9
11
12
13
14
15
16
17
18
19
20
21
22
24
27
29
30
31
32
34
39
40
41
44
47
48
51
54
58
61
1
1
O
2
2
1
O
3
3
2
2
1
1
O
2
2
1
1
2
2
3
3
4
4
4
5
5
5
6
6
6
7
7
8
9
9
9
10
10
12
12
12
13
14
14
15
16
17
18
2
1
3
2
1
4
4
2
1
3
2
3
5
5
3
4
5
6
6
7
8
9
9
10
11
11
12
13
13
14
15
15
17
19
20
21
22
22
24
27
28
29
31
33
34
36
38
41
43
14332.35
17059.88
22783.16
23544.17
24305.05
28319.46
29630.76
31737.04
31843.12
31238.16
32998.52
33668.40
35135.77
36141.48
30736.20
33398.34
27246.46
34940.21
23392.26
31435.20
25028.03
33760.09
17334.45
25274.60
34531.34
16466.01
24450.14
34012.44
15068.20
22843.75
32458.44
13381.85
30125.46
27267.87
15853.17
24126.67
34714.27
13457.60
30811.78
14915.51
22939.98
,3611.15
28915.87
24483.16
35768.37
30487.96
25610.22
31608.59
26377.81
Centr.
-
Obs.
eale.
(MHz)
. O5
-.09
. O3
.13
-.10
.06
.13
.13
-.01
-.12
-.08
.01
- .12
.00
.14
-.02
-.01
-.07
-.09
-.07
-.08
.00
- .11
-.09
.08
-.05
-.17
.07
-.02
-.01
.14
.07
.10
. O5
. 03
.04
.07
. O5
. 07
. 02
-.13
.00
-.05
-.02
-.07
-.14
.01
.05
.08
eorr.
(MHz)
-.01
-.07
. O3
-.23
-.27
. O6
.07
-.72
-.74
-.28
-.39
- .16
.14
.15
-.84
-1 .29
-1 .26
-1 .95
-2.44
-3.72
- 5.17
-7.59
-5.78
-8.82
-12.75
-8.66
-13.12
-18.88
-11.68
-17.73
-25.58
-14.59
-32.37
-38.78
-29.88
-44.39
-63.04
-32.47
-70.37
-53.76
-79.70
-113.44
-117.70
-119.24
-168.49
-169.71
-167.50
-231.92
-224.24
..05 MHz
First
1
2
2
ex.
1
O
2
1
2
1
2
3
3
3
3
3
3
4
4
7
8
9
11
12
14
15
17
18
20
21
24
27
30
31
34
40
41
44
47
51
2
1
O
2
3
2
1
1
O
1
2
2
3
3
4
4
5
5
6
6
7
8
9
9
10
12
12
13
14
15
"LOS
Seeond ex.
2
3
3
3
4
4
11
12
14
15
17
18
20
24
30
34
41
44
47
2
3
3
1
O
2
state of CHO-COOH
O
1
O
1
1
O
3
3
4
4
5
5
6
7
9
10
12
13
14
"..10
2
3
2
14342.18
22786.71
23545.33
.12
.11
. 00
-. O2
.00
-.22
3
3
3
4
4
"L15
3
4
4
4
4
4
4
5
5
7
8
9
11
12
14
15
17
18
20
21
24
27
30
31
34
40
41
44
47
51
2
1
O
2
3
2
1
1
O
1
2
2
3
3
4
4
5
5
6
6
7
8
9
9
10
12
12
13
14
15
1
4
4
3
2
2
3
5
5
6
6
7
8
9
10
11
12
13
14
15
17
19
21
22
24
28
29
31
33
36
24304.18
28339.98
29638.24
31240.33
31737.54
32994.59
33653.27
35162.57
36156.17
34690.93
23272.54
31260.21
24947.13
33625.87
25248.89
34459.31
24487.74
34016.78
22944.80
32545.31
30293.22
27507.73
24423.21
35051.90
31220.28
23422.27
34211.08
29549.16
25122.36
31307.45
-.2
-. OS
-. O
-. O
-.2
.04
-. O5
-.06
- .'04
-.08
.06
.01
-.01
-.03
.07
-.6
-.4
-.2
-. O
-. O
-1 .5
-2.2
-.02
. O3
-.03
.01
.03
- .13
.06
- .14
.01
-.07
.17
.04
.07
.00
.04
-.04
-.18
.03
.06
-3.3
-4.9
-7.1
-8.5
-12.2
-12.9
-18.4
-17.6
-25.2
- 32.2
-38.9
-44.8
-63.3
-71.1
-81.5
-115.4
-120.5
-122.7
-174.8
MHz
2
3
3
2
4
4
9
10
11
12
13
14
15
18
22
25
30
32
34
C-C torso state of CHO-COOH
3
4
4
4
5
5
11
12
14
15
17
18
20
24
30
34
41
44
47
O
1
O
1
1
O
3
3
4
4
5
5
6
7
9
10
12
13
14
3
4
4
3
5
5
8
9
10
11
12
13
14
17
21
24
29
31
33
22786.81
28353.63
29641.17
33635.28
35180.70
36164.02
24366.16
33496.80
25211.24
34378.75
24500.72
33996.77
23009.53
30405.67
24642.66
31523.35
34664.04
30033.65
25615.65
-.1
.26
-.17
.11
.07
-.01
-.2
-.2
-.3
-.4
-.4
- .16
.20
-.10
.07
-. O2
-.15
-.04
-4.3
-6. a
-8. a
-11.1
-12.5
-17.5
-17.5
-.05
- 32.2
.06
.08
-46.3
-.06
-73.8
-.02-121.7
.10 -128.5
-.08
-132.3
MHz
Third
C-C tors.
O
3
3
2
1
1
2
4
4
7
7
8
9
10
11
12
13
14
15
16
18
20
22
23
25
29
30
32
34
37
ex.
1
O
1
1
O
3
3
2
4
4
MHz
C-C torso
4
4
4
5
5
1
O
1
1
O
4
4
3
5
5
state of CHO-COOH
28364.87
29642.03
33623.53
35195.74
36169.90
.28
-.64
.11
.23
.41
143
TABLE
2: continued
First ex. in-planebending mode of
2
2
3
3
3
3
3
3
4
4
8
9
11
12
14
15
17
18
20
21
24
27
30
31
37
40
41
44
O
2
1
O
2
3
2
1
1
O
2
2
3
3
4
4
5
5
6
6
7
8
9
9
11
12
12
13
".10
2
O
3
3
2
O
1
2
4
4
7
8
9
10
11
12
13
14
15
16
18
20
22
23
27
29
30
32
3
3
4
4
4
4
4
4
5
5
8
9
11
12
14
15
17
18
20
21
24
27
30
31
37
40
41
44
O
2
1
O
2
3
2
1
1
O
2
2
3
3
4
4
5
5
6
6
7
8
9
9
11
12
12
13
3
1
4
4
3
1
2
3
5
5
6
7
8
9
10
11
12
13
14
15
17
19
21
22
26
28
29
31
22748.21
24286.57
28262.01
29576.83
31200.87
31812.65
32979.03
33645.41
35061.56
36067.39
23584.65
31677.54
25288.44
34082.34
25602.15
34936.28
24839.37
34498.15
23283.77
33017.66
30745.30
27930.14
24810.15
35594.47
27729.62
23820.60
34777.76
30054.74
Ground
CHO-COOH
.12
.00
.03
- .03
.04
-.07
-.04
.09
.03
-.12
.04
.06
.03
-.02
- .12
- .01
- .08
-.09
.08
.02
.13
-.01
.01
.01
- .06
.00
- .01
.03
2
2
2
2
3
3
3
3
3
3
3
4
4
11
17
23
26
29
32
35
42
45
- .01
-.27
-.03
- .01
-.30
-.70
- .41
-.20
-. O3
-.03
-2.27
-3.40
-5.04
-7.32
-8.82
-12.62
-13.33
.19.02
-18.24
-26.12
-33.42
-40.44
-46.72
-65.94
-80.92
-85.57
-121.10
-126.72
O
2
2
1
1
O
2
3
3
2
1
1
O
3
5
7
8
9
10
11
13
14
"..05
state of CHO-COOD
2
1
O
1
3
3
2
1
O
1
2
4
4
9
13
17
19
21
23
25
30
32
3
3
3
3
4
4
4
4
4
4
4
5
5
11
17
23
26
29
32
35
42
45
O
2
2
1
1
O
2
3
3
2
1
1
O
3
5
7
8
9
10
11
13
14
3
2
1
2
4
4
3
2
1
2
3
5
5
8
12
16
18
20
22
24
29
31
22502.61
23372.76
24242.72
25333.27
27950.64
29179.96
30986.24
31555.58
31691.79
32967.68
33455.94
34646.45
35533.30
28192.25
31084.29
30677.13
29587.22
28076.49
26262.56
24252.81
30215.63
27447.88
. O5
.01
-.14
-.07
- .16
- .08
.05
.10
.07
-. O3
.06
.09
.05
-.03
- .02
.07
. O5
.00
-.07
-.01
-.06
.06
-.12
- .19
-.23
- .16
-.31
-.30
-.35
-.55
-.57
-.45
-.33
-.62
-.62
-3.69
-10.78
-18.89
-22.40
-25.13
-26.92
-27.67
-4 0.36
-37.51
MHz
MHz
TAB
LE
3
CORRELATION
MATRIX
FOR THE ROTATIONAL
GROUND VIBRATIONAL
B'
A'
1.000
0.301
-0.266
0.577
0.649
0.603
-0.406
AND
CENTRIFUGAL
DISTORTION
CONSTANTS OF THE
STATE OF CHO-COOH
C'
1.000
0.729
0.047
0.038
0.050
-0.077
1.000
-0.558
-0.566
-0.568
0.525
T' aaaa
T' bbbb
T' eeee
T' aabb
1.000
0.989
0.992
-0.957
1.000
0.994
-0.914
1.000
-0.947
1.000
TABLE 4
MOLECULAR
CONSTANTS FOR CHO-COOH
Vibrational state
Number of transitians
a (MHz)
CHO-COOH
Av (MHz)
Bv (MHz)
Cv (MHz)
la (UA2)
Ib (UA2)
le (UA2)
T'aaaa(kHz)
T'bbbb(kHz)
T'eeee(kHz)
T'aabb(kHz)
Ie-Ia-Ib
(pA2)
AND CHO-COOD
Ground
First ex. C-C tors
49
0.082
33
0.073
10966.813 :1::0.015
4605.988 :1::0.003
3242.092 :1::0.004
46.08230:1::0.00006
109.72151 :1::0.00007
155.87960 :1::0.00020
-39.7
-3.31
+1.8
-36.79
:1::5.8
:1::0.16
:1::0.4
:1::0.76
0.07578 :1::0.00022
10893.489 :1::0.052
4601.746 :1::0.003
3246.763 :1::0.007
46.39248 :1::0.00022
109.82266 :1::0.00007
155.65536:1::0.00035
-62.0
:1::12.1
-4.00
:1::0.37
+0.06
:1::0.8
-33.5
:1::1.2
- 0.55978 :1::0.00042
Second ex. C-C torso
19
0.116
10830.244
:1::0.209
4597.379
:1::0.008
3250.386
:1::0.029
46.66340:1::0.00089
109.92699:1::0.00017
155.4818 :1::0.0015
-131.8
:1::39.5
-6.1
:I::1.3
-5.1
:1::2.8
-24.0
:1::4.0
-1.1085
:1::0.0017
144
TABLE 4 (continued)
Vibratianal state
Third ex. C-C tars.
First ex. in-plane bend
5
28
0.062
Number aftransitians
(J (MHz)
Av (MHz)
Bv (MHz)
Cv (MHz)
la (UÅ2)
lb (UÅ 2)
le (UÅ 2)
T' aaaa(kHz)
T' bbbb(kHz)
T' eeee (kHz)
T' aabb(kHz)
le-la-lb
(UA2)
Conversion
factor
The uncertainties
VIBRATIONAL
10773.86
4594.44
3253.18
46.908
109.9973
155.3483
::1:0.057
::1:0.13
::1:0.08
::1:0.014
::1:0.0028
::1:0.0040
-1.557
::1:0.015
10976.945
::1:0.057
4606.017
::1:0.003
3233.132
::1:0.007
46.03977 ::1:0.00024
109.72083 ::1:0.00007
156.31158 ::1:0.00035
-66.3
::1:11.6
-4.17
::1:0.37
-0.1
::1:0.8
-33.7
::1:1.3
0.55099::1:0.00044
Ground
22
0.071
CHO-COOD
10422.262
::1:0.136
4600.668
::1:0.004
3190.305
::1:0.010
48.49005::1: 0.00063
109.84839::1:0.00009
158.40993 ::1:0.00050
-138.6
::I:12.2
-6.45
::1:0.55
-6.5
::1:1.0
-23.3
::1:2.6
0.07149::1:0.00078
505376 UA2 MHz.
represent
one standard
deviation.
SATELLITE SPECTRA
Several sets of satellite lines were found accompanying the ground state
transitions. As shown in Table 2, these satellites have been assigned to excited states
of the two lowest normal vibrational modesl, the C-C torsion and the lowest
skeietal bending made. All of the satellite lines are narrow, unsplit lines, and they
fit the first order perturbation expression of eqn. (l). The ca1culated frequencies
of Table 2 were obtained from the rotational and centrifugal distortion constants
given in Table 4, with the exception of the third excited state of the C-C torsion
where no allowance for centrifugal distortion has been made. A few transitions
of the fourth excited state of this made were also observed, but the rotational
constants were not obtained.
The strongest satellite, about 40 % as intense as the ground state line, is
assigned to the first excited state of the C-C torsion. The satellites attributed to
its overtone states lie in a series of steadily decreasing intensity. To the first approximation, the inerti al defect LIdepends on the vibrational state according to
LI = ILls(vs+-!-).
(6)
The observed change in LI= Lllex.C-Ctors- Llgroundis negative, viz., LI ~ - 0.6 uÅ 2.
This is consistent with the assignment of this vibration as the lowest out-of-plane
mode2l, i.e. presumably the C-C torsional made.
Another
satellite having about 20
% of the intensity of the ground state line
is assigned to the first excited state of the skeIetal bending vibration. In this case
145
TABLE 5
RELATIVE INTENSITIES'
ACID
AND ENERGY DIFFERENCES
Transition
Relative intensity
31,3
->-
41.4
41,4
->-
51,5
185,14
->- 185,13
206,15
->- 206,14
31,3
->-
41,4
41,4
->-
51,5
206,15
OF VIBRATIONALLY
Energy difference
c-c
torso ground state
0.41
0.44
0.46
0.47
Av:
0.445 ::1::0.027
167::1::12 cm-l
In-plane bend ground
0.29
0.24
0.21
->- 206,14
EXCLTED STATES OF GL YOXYLIC
state
Av: 0.247::1::0.033
288::1::26 cm-l
.
The uncertainties represent one standard deviation.
T = 296 oK.
LIis positive, LI ~ +0.5, in agreement with its assignment to a low frequency inplane mode21.
Relative intensity measurements were perforrned at room temperature.
Most but not all of the precautions of ref. 22 were observed. The results are
presented in Table 5. The energy differences between states are derived by assuming
that the relative intensity is proportional to the Boltzmann facto r. A C-C torsional
frequency of 167 cm-1 with a standard deviation of 12 cm-1 should be compared
to 122 cm -1 deduced from an infrared combination model. The lowest in-plane
mode of 288:t26 cm-1 is also somewhat different from 365 cm-1 observed by
Raman spectroscopy1 of an aqueous solution of glyoxylic acid.
The inerti al defect can be used to estimate the energy of the C-C torsional
mode. Oka and Morino23 have shown that
LI ~ LlYib=
I
(~
n c)(nv+t) I
v' Wv
Wv - Wv'2)[(,~~!)2+(,~~!)2_('~~!?J
(~;.
+
+
I
t
where
Wt
=
Wv for an out-of-plane
vibration,
(~ )(~ )(nt+t)
n c
(7)
2Wt
Wt = 00 for an in-plane vibration,
Wv is the fundamental frequency (in cm -1) of the vibrational mod e considered
and Wv' represents the frequencies of all the other vibrational fundamentals with
which it interacts. " s are the Coriolis coupling constants between interacting states.
The z axis has been chosen perpendicular to the molecular plane which contains
the x and y axes. From eqn. (7), the general expression for the change of inertial
146
defect in successive vibrational states can be written as
(jLl =
Llv+ 1
-
LIv
h
3
" w;,
= -+L...,
n2c ){ 2wt
v' wvCw;-w;,)
(
X
[((~~~)2+((~~)2_((~~)2]}.
(8)
Simple arguments show that if an in-plane mode interacts with an out-ofplane mode, then (vv,(X) "# O, (v/Y) "# O, and (vv'(z)
do not interact and therefore
= O.Two out-of-plane modes
= (vv'(Y) = (vv'(z) = O,
Consequently the
torsional mode can interact only with an in-plane-mode and in the limit that the
frequency of the latter tends to infinity, eqn. (8) reduces to
(jLl = -
h
(vv'(x)
.
2n2clwtl
(9)
Substituting the observed value of (jLl~ -0.6 uÅ 2 in eqn. (9) the torsional
frequency of 112 cm -1 is calculated. A lowering of the calculated value from the
observed 167 cm-1 is expected because of the finite frequency of the in-plane
modes with which the torsional mode interacts. The lowering of as much as about
50 cm-
1
seemsto be somewhatmore than what is usually found.
As can be seen from Table 4, the rotational constants of successively excited
states of the c-c torsion varies regularly, although not linearly. No zigzagging
behaviour is observed as one would expect for a molecule having a potential hump
at the planar position24. These excited states thus constitute strong evidence for
a plan ar equilibrium configuration of glyoxylic acid.
The centrifugal distortion perturbation of the excited vibrational states is
seen to be remarkably similar to that of the ground state (Table 2). High correlations among the -r's, similar to those for the ground vibrational state, exist also
for the -r's of the excited vibrational states.
DlPOLE
MOMENT
The Stark coefficients of the 22,0 --+ 32,1 and 31,3 --+ 41,4 transitions
were
used to determine the dipole moment. A d.c. voltage was applied between the
Stark septum and the cell, with the modulating square wave voltage superimposed.
The d.c. voltage was measured with a digital voltmeter having an accuracy of
0.025%. The electric field was calibrated using the OCS 1 --+ 2 transition with
/locs = 0.71521 D (ref. 25). Table 6 gives the results with the derived dipole
moments and its components along the principal axes. /le was assumed to be zero
in the calculations which were carried out using the computer programme MB04
described previously26.
147
TABLE 6
STARK COEFFICIENTS
AND DIPOLE
MOMENT OF GLYOXYLIC
Transition
22.0 ->- 32.1
31.3 ->- 41.4
ACID
/j.v/E2
(MHz (V/cm)-2)
X 106
Observed
Calculated
7.51
3.23
7.83
M=O
M=2
M=3
7.44
3.18
7.82
/-l. = 1.85 :1::0.03
/-lb= 0.20:1::0.10
/-l = 1.86:1::0.04
There is fair agreement between the dipole moment components obtained
by vectorial addition of bond moments, viz. /la = 1.8D, and /lb = 0.6 D, and the
experimentally determined ones, viz. /la = 1.85::1:0.03D, and /lb = 0.20::1:0.10D.
Theresults obtained by the CNDOj2computations were/la = 2.3 D and /lb = 0.7 D.
MICROWAVE SPECTRUM OF CHO-COOD
AND THE CONFORMATION OF GLYOXYLIC
ACID
The microwave spectrum of CHO-COOD was studied mainly to decide
definitely whether glyoxylic acid exists as trans l or trans 2. Search was made
initially for the intense J -+ J + I, AK- 1 = O,AK1 = + l series and the lines were
found within less than 50 MHz from the frequencies predicted for the trans l
conformation. No lines which could be attributed to trans 2 were seen in the spectrum.
The assignment of the high J Q-branch transitions proceeded in much the
same way as for the parent species. The frequencies were fitted to eqn. (1) and the
rotational and centrifugal distortion constants are presented in Table 2.
Kraitchman's equations27 may be used to locate the hydroxyl hydrogen
coordinates directly using the rotational constants of CHO-COOH and
CHO-COOD. With the former species as the parent molecule, lal = 0.3477 Å,
and jbl = 1.5581 Å, are ca1culated. These coordinates are dose to lal = 0.531 Å
and Ibl = 1.493 Å ca1culated from the plausible structural parameters of Table l
for the trans l conformation
and
strikingly
different
from lal
=
1.559 Å and
Ibl = 1.522 Å computed for trans 2 from the same structural parameters with the
exception of the hydroxyl hydrogen position. Hence, the most stable conformation
of glyoxylic acid is undoubtedly trans 1. A mode! of the molecule in the principal
axis system is depicted in Fig. 2. Accurate structural parameters cannot be derived
from the two isotopic molecules reported here and a study of more isotopic speeies
of glyoxylic acid is planned.
148
6
ly
o
o
Q
c
o
ly
Fig. 2. Projection
of stable conformation
of glyoxylic
acid in the a-b principal
axes pl ane.
The existenceof additional conformations is of considerable interest. A
thorough searchhasbeenmadein the 22-36.3GHz region,but no unassignedlow J
lines not attributable to trans l were observed. In this spectral region virtually
all high and medium intensity lines have been assigned.Due to the comparatively
simple nature of the spectrum and the large number of assigned lines it is estimated
that concentrations of additional conformations other than trans l exceeding a
total of 10 % would have been detected. This number is a conservativeestimate
and it is felt that even 5 % concentrations of rota mers with high dipole moments
would have been noticed. If the safe 10 % existence of additional conformations is
accepted and used to estimate the energy difference between trans l and other
rotamers assuming Boltzmann distribution, then the energy difference between
trans 1 and conformers with a statistical weight of 1, i.e. other trans or ds conformers, is estimated to be at least 1.3 kcal mol-l. For gauche and skew rota mers
and other forms having a statistical weight of 2, the trans l form is at least 1.7 kcal
mol-l more stable.
DISCUSSION
The reason why free glyoxylicacid prefers a planar trans 1 configurationis
pro babl y complex. The two effects conjugation and the five member intramolecular
hydrogen bond should both stabilize the observed conformation. It is very difficult
to estimate quantitatively how much each of these effects contributes, but the
149
hydrogen bond is presurnably the most important of the two. Same indication of
the strength of the hydrogen bond may be inferred from the non-bonded O . . . O
distance. Using the plausible structure of Table l, this length is ca1culated to be
2.65 Å indicating a hydrogen bond strength in the order of 2-5 kcal mol- 1.
Another information about the stabil ity of the hydrogen bond is also available from
the infrared work. The O-H stretching vibration for glyoxylic acid1 is reported to
be 3510 cm -1 intermediate between 3475 cm -1 observed for oxalic acid6 and
3570 cm-l found for monomeric formic acid28. Hence, the hydrogen bon d seems
to be slightly weaker in this case than in oxalic acid, but stronger than in formic
acid.
The importanee of conjugation in glyoxylic acid is also very hard to estimate,
because accurate structural parameters are not as yet available. However, for the
cIosely related substances glyoxal15 and oxalic acid6 rather long C-C single bonds
of 1.525 Å and 1.548 Å, respectively, have been determined indicating that conjugation is not very important for this type of compounds.
The question why the five-member hydrogen bond of trans I is preferred to
the four member ring of trans 2 is probably a resuIt of the more favourable geometrical situation in the former case. From the structural parameters of Table l
it is ca1culated that the non-bonded H. . . O distances are roughly 1.97 Å for
trans 1 and 2.29 Å for trans 2, respectively. The hydroxyl proton is thus cIoser to
the carbonyl oxygen by about 0.3Å in the former case allowing a stronger hydrogen
bond to be formed.
ACKNOWLEDGEMENT
A preliminary study of this problem was made by one of us (H. M.) at the
University of Texas at Austin, U.S.A., and H. M. would like to express his sincere
gratitude to Professor James E. Boggs and his group. He is also grateful to the
U.S. State Department for Fulbright travel grant, to the Robert A. Welch Foundation and the University of Texas for support.
The Norwegian Research Council for Science and the Humanities is thanked
for financial support.
REFERENCES
l
2
3
4
G.
G.
A.
L.
FLEURY AND V. TABACIK, J. Mol. Structure,
10 (1971) 359, erratum 12 (1972) 156.
H. KWEI AND R. F. CURL JR., J. Chem. Phys., 32 (1960) 1592.
ALMENNINGEN, O. BASTIANSEN AND T. MOTZFELDT, Acta Chem. Scand., 23 (1969) 2848.
C. KRISHER AND E. SAEGEBARTH,J. Chem. Phys., 54 (1971) 4553.
5 B. P. VAN EUCK, G. VAN DER PLAATS AND P. H. VAN ROON, J. Mol. Structure, Il (1972) 67.
6 Z. NAHLOVSKA, B. NAHLOVSKY AND T. G. STRAND, Acta Chem. Scand., 24 (1970) 2617.
7 G. N. CURRIE AND D. A. RAMSAY, Can. J. Phys., 49 (1971) 317.
150
8 K. HEDBERG, Fourth Austin Symposium on Gas Phase Molecular Structure, (Austin 1972), p. 72.
9 K.-M. MARSTOKK AND H. MØLLENDAL, J. Mol. Structure, 5 (1970) 205.
10 M. S. CORD, J. D. PETERSEN, M. S. LOJKO AND R. H. HAAS, Microwave Spectral Tables Vo/. IV,
Nat. Bur. Stand., Boulder, Colo., p. 75.
11 J. M. DOWLING, J. Mol. Speetrosc., 6 (1961) 550.
12 G. W. KING, R. M. HAINER AND P. C. CROSS, J. Chem. Phys., 11 (1942) 27.
13 R. L. COOK, J. Chem. Phys., 42 (1965) 2927.
14 W. H. KIRCHHOFF, J. Mol. Speetrosc., 41 (1972) 333.
15 K. KUCHITSU, T. FUKAYAMA AND Y. MORINO, J. Mol. Structure, 1 (1967/68) 463.
16 C. P. SMYTH, Dielectric Behavior and Structure, McGraw-Hill,
New York, 1955, p. 244.
17 J. A. POPLE AND D. L. BEVERIDGE, Approximate
Molecular
Orbital Theory, McGraw-Hill,
New York, 1970.
18 J. A. POPLE AND M. GORDON, J. Amer. Chem. Soc., 89 (1967) 4253.
19 O. GROPEN AND H. M. SEIP, Chem. Phys. Lett., 11 (1971) 445.
20 D. KIVELSON AND E. B. WILSON JR., J. Chem. Phys., 21 (1953) 1229.
21 D. R. HERSCHBACH AND V. W. LAURIE, J. Chem. Phys., 40 (1964) 3142.
22 A. S. ESBITT AND E. B. WILSON JR., Rev. Sei. Instrum., 34 (1963) 901.
23 T. OKA AND Y. MORINO, J. Mol. Speetrosc.,
6 (1961) 472.
24 S. J. CHAN, J. ZINN, J. FERNANDEZ AND W. D. GWINN, J. Chem. Phys., 33 (1960) 1643.
25 J. S. MUENTER, J. Chem. Phys., 48 (1968) 4544.
26 K.-M. MARSTOKK AND H. MØLLENDAL, J. Mol. Structure, 4 (1969) 470.
27 J. KRAITCHMAN, Amer. J. Phys., 21 (1953) 17.
28 R. C. MILLIKAN AND K. S. PITZER, J. Chem. Phys., 27 (1957) 1305.