Effects of hydrogel properties and extrusion parameters on 3D bioprinting
advertisement
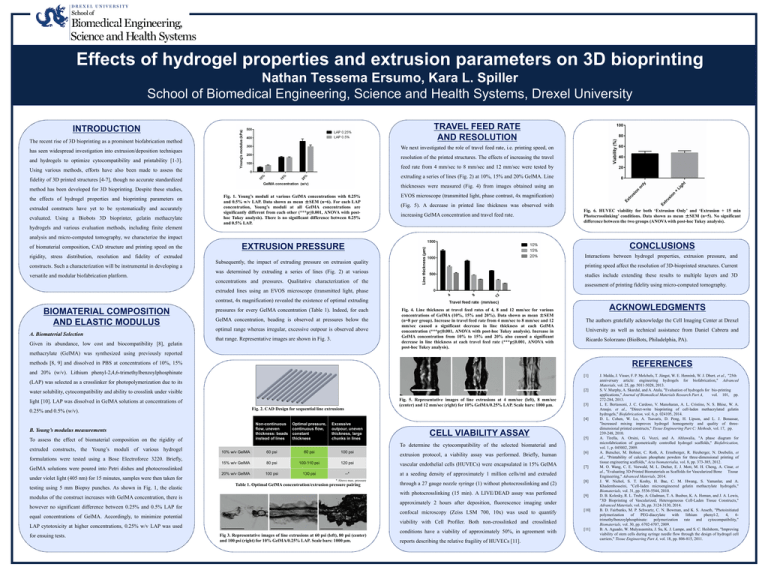
Effects of hydrogel properties and extrusion parameters on 3D bioprinting Nathan Tessema Ersumo, Kara L. Spiller School of Biomedical Engineering, Science and Health Systems, Drexel University TRAVEL FEED RATE AND RESOLUTION INTRODUCTION The recent rise of 3D bioprinting as a prominent biofabrication method We next investigated the role of travel feed rate, i.e. printing speed, on has seen widespread investigation into extrusion/deposition techniques resolution of the printed structures. The effects of increasing the travel and hydrogels to optimize cytocompatibility and printability [1-3]. feed rate from 4 mm/sec to 8 mm/sec and 12 mm/sec were tested by Using various methods, efforts have also been made to assess the extruding a series of lines (Fig. 2) at 10%, 15% and 20% GelMA. Line fidelity of 3D printed structures [4-7], though no accurate standardized thicknesses were measured (Fig. 4) from images obtained using an method has been developed for 3D bioprinting. Despite these studies, the effects of hydrogel properties and bioprinting parameters on extruded constructs have yet to be systematically and accurately evaluated. Using a Biobots 3D bioprinter, gelatin methacrylate hydrogels and various evaluation methods, including finite element Fig. 1. Young’s moduli at various GelMA concentrations with 0.25% and 0.5% w/v LAP. Data shown as mean ±SEM (n=6). For each LAP concentration, Young’s moduli at all GelMA concentrations are significantly different from each other (***p≤0.001, ANOVA with posthoc Tukey analysis). There is no significant difference between 0.25% and 0.5% LAP. EVOS microscope (transmitted light, phase contrast, 4x magnification) (Fig. 5). A decrease in printed line thickness was observed with increasing GelMA concentration and travel feed rate. Fig. 6. HUVEC viability for both ‘Extrusion Only’ and ‘Extrusion + 15 min Photocrosslinking’ conditions. Data shown as mean ±SEM (n=5). No significant difference between the two groups (ANOVA with post-hoc Tukey analysis). analysis and micro-computed tomography, we characterize the impact of biomaterial composition, CAD structure and printing speed on the rigidity, stress distribution, resolution and fidelity of extruded constructs. Such a characterization will be instrumental in developing a versatile and modular biofabrication platform. CONCLUSIONS EXTRUSION PRESSURE Interactions between hydrogel properties, extrusion pressure, and Subsequently, the impact of extruding pressure on extrusion quality printing speed affect the resolution of 3D-bioprinted structures. Current was determined by extruding a series of lines (Fig. 2) at various studies include extending these results to multiple layers and 3D concentrations and pressures. Qualitative characterization of the assessment of printing fidelity using micro-computed tomography. extruded lines using an EVOS microscope (transmitted light, phase contrast, 4x magnification) revealed the existence of optimal extruding BIOMATERIAL COMPOSITION AND ELASTIC MODULUS A. Biomaterial Selection Given its abundance, low cost and biocompatibility [8], gelatin pressures for every GelMA concentration (Table 1). Indeed, for each GelMA concentration, beading is observed at pressures below the optimal range whereas irregular, excessive outpour is observed above that range. Representative images are shown in Fig. 3. Fig. 4. Line thickness at travel feed rates of 4, 8 and 12 mm/sec for various concentrations of GelMA (10%, 15% and 20%). Data shown as mean ±SEM (n=8 per group). Increase in travel feed rate from 4 mm/sec to 8 mm/sec and 12 mm/sec caused a significant decrease in line thickness at each GelMA concentration (***p≤0.001, ANOVA with post-hoc Tukey analysis). Increase in GelMA concentration from 10% to 15% and 20% also caused a significant decrease in line thickness at each travel feed rate (***p≤0.001, ANOVA with post-hoc Tukey analysis). ACKNOWLEDGMENTS The authors gratefully acknowledge the Cell Imaging Center at Drexel University as well as technical assistance from Daniel Cabrera and Ricardo Solorzano (BioBots, Philadelphia, PA). methacrylate (GelMA) was synthesized using previously reported methods [8, 9] and dissolved in PBS at concentrations of 10%, 15% REFERENCES and 20% (w/v). Lithium phenyl-2,4,6-trimethylbenzoylphosphinate [1] (LAP) was selected as a crosslinker for photopolymerization due to its [2] water solubility, cytocompatibility and ability to crosslink under visible Fig. 5. Representative images of line extrusions at 4 mm/sec (left), 8 mm/sec (center) and 12 mm/sec (right) for 10% GelMA/0.25% LAP. Scale bars: 1000 µm. light [10]. LAP was dissolved in GelMA solutions at concentrations of Fig. 2. CAD Design for sequential line extrusions 0.25% and 0.5% (w/v). [3] [4] Non-continuous flow, uneven thickness: beads instead of lines B. Young’s modulus measurements To assess the effect of biomaterial composition on the rigidity of extruded constructs, the Young’s moduli of various hydrogel formulations were tested using a Bose Electroforce 3220. Briefly, Optimal pressure, continuous flow, constant thickness Excessive outpour, uneven thickness, large chunks in lines testing using 5 mm Biopsy punches. As shown in Fig. 1, the elastic 10% w/v GelMA 60 psi 80 psi 100 psi extrusion protocol, a viability assay was performed. Briefly, human 15% w/v GelMA 80 psi 100-110 psi 120 psi vascular endothelial cells (HUVECs) were encapsulated in 15% GelMA 20% w/v GelMA 100 psi 130 psi --* at a seeding density of approximately 1 million cells/ml and extruded * Above max. pressure Table 1. Optimal GelMA concentration/extrusion pressure pairing through a 27 gauge nozzle syringe (1) without photocrosslinking and (2) with photocrosslinking (15 min). A LIVE/DEAD assay was perfomed modulus of the construct increases with GelMA concentration, there is [6] [7] [8] [9] approximately 2 hours after deposition, fluorescence imaging under however no significant difference between 0.25% and 0.5% LAP for confocal microscopy (Zeiss LSM 700, 10x) was used to quantify equal concentrations of GelMA. Accordingly, to minimize potential [10] viability with Cell Profiler. Both non-crosslinked and crosslinked LAP cytotoxicity at higher concentrations, 0.25% w/v LAP was used for ensuing tests. [5] To determine the cytocompatibility of the selected biomaterial and GelMA solutions were poured into Petri dishes and photocrosslinked under violet light (405 nm) for 15 minutes, samples were then taken for CELL VIABILITY ASSAY Fig 3. Representative images of line extrusions at 60 psi (left), 80 psi (center) and 100 psi (right) for 10% GelMA/0.25% LAP. Scale bars: 1000 µm. conditions have a viability of approximately 50%, in agreement with reports describing the relative fragility of HUVECs [11]. [11] J. Malda, J. Visser, F. P. Melchels, T. Jüngst, W. E. Hennink, W. J. Dhert, et al., "25th anniversary article: engineering hydrogels for biofabrication," Advanced Materials, vol. 25, pp. 5011-5028, 2013. S. V. Murphy, A. Skardal, and A. Atala, "Evaluation of hydrogels for bio‐printing applications," Journal of Biomedical Materials Research Part A, vol. 101, pp. 272-284, 2013. L. E. Bertassoni, J. C. Cardoso, V. Manoharan, A. L. Cristino, N. S. Bhise, W. A. Araujo, et al., "Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels," Biofabrication, vol. 6, p. 024105, 2014. D. L. Cohen, W. Lo, A. Tsavaris, D. Peng, H. Lipson, and L. J. Bonassar, "Increased mixing improves hydrogel homogeneity and quality of threedimensional printed constructs," Tissue Engineering Part C: Methods, vol. 17, pp. 239-248, 2010. A. Tirella, A. Orsini, G. Vozzi, and A. Ahluwalia, "A phase diagram for microfabrication of geometrically controlled hydrogel scaffolds," Biofabrication, vol. 1, p. 045002, 2009. A. Butscher, M. Bohner, C. Roth, A. Ernstberger, R. Heuberger, N. Doebelin, et al., "Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds," Acta biomaterialia, vol. 8, pp. 373-385, 2012. M. O. Wang, C. E. Vorwald, M. L. Dreher, E. J. Mott, M. H. Cheng, A. Cinar, et al., "Evaluating 3D‐Printed Biomaterials as Scaffolds for Vascularized Bone Tissue Engineering," Advanced Materials, 2014. J. W. Nichol, S. T. Koshy, H. Bae, C. M. Hwang, S. Yamanlar, and A. Khademhosseini, "Cell-laden microengineered gelatin methacrylate hydrogels," Biomaterials, vol. 31, pp. 5536-5544, 2010. D. B. Kolesky, R. L. Truby, A. Gladman, T. A. Busbee, K. A. Homan, and J. A. Lewis, "3D Bioprinting of Vascularized, Heterogeneous Cell‐Laden Tissue Constructs," Advanced Materials, vol. 26, pp. 3124-3130, 2014. B. D. Fairbanks, M. P. Schwartz, C. N. Bowman, and K. S. Anseth, "Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2, 4, 6trimethylbenzoylphosphinate: polymerization rate and cytocompatibility," Biomaterials, vol. 30, pp. 6702-6707, 2009. B. A. Aguado, W. Mulyasasmita, J. Su, K. J. Lampe, and S. C. Heilshorn, "Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers," Tissue Engineering Part A, vol. 18, pp. 806-815, 2011.




