Chemistry Chapter 13 Raymond Chang 10
advertisement
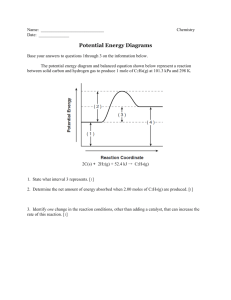
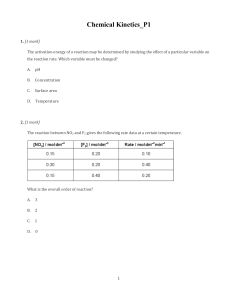
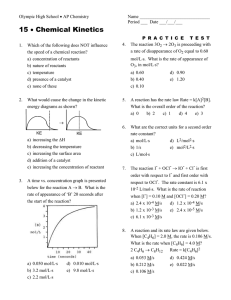
Chemistry Raymond Chang 10th edition Chapter 13 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Question 1 For the reaction 2A + B → C it is found that changing the concentration of A has no impact on the rate, but when B is doubled the rate doubles. The rate law is 25% 25% 25% 2 3 25% A) rate = k[A]2[B]. B) rate = k[A][B]2. C) rate = k[B]2. D) none of the above. 1 4 Question 2 If the activation energy of a reaction is decreased, the reaction rate will increase. A) True B) False 50% 50% 1 2 Question 3 Which of the following is true? 20% 20% A) A catalyst is not consumed in the reaction. B) A catalyst speeds up the forward reaction. C) A catalyst speeds up the reverse reaction. D) A catalyst decreases the activation energy. E) All of the above. 1 2 20% 3 20% 4 20% 5 Question 5 Which of the following will cause the reaction rate to increase? 20% 20% 20% 2 3 20% 20% A) Increasing the temperature. B) Adding a catalyst. C) Reducing the concentration of reactants. D) Both A and B. E) Both B and C. 1 4 5 Question 6 Using the data below, determine the rate law for the reaction of A with B to produce C. Exp 1: 0.100 M A, 0.100 M B, rate 0.120 M/s Exp 2: 0.100 M A, 0.200 M B, rate 0.120 M/s Exp 3: 0.200 M A, 0.100 M B, rate 0.480 M/s 25% 25% 25% 2 3 25% A) rate=k[A]2[B] B) rate=k[A]2 C) rate=k[B]2 D) rate=k[A][B] 1 4 Question 8 If a proposed mechanism is consistent with the experimentally determined rate law, then the mechanism must be correct. A) True B) False 50% 50% 1 2 Question 9 If a reaction is exothermic, then the activation energy for the forward reaction is __________ the activation energy for the reverse reaction. 25% 25% 25% 2 3 25% A) greater than B) less than C) equal to D) none of the above--there is no relationship between the values 1 4 Question 10 Reactions rarely occur by mechanisms that include trimolecular elementary steps because 25% 25% 25% 2 3 25% A) the simultaneous collision of three molecules has a low probability. B) simpler reaction mechanisms can always support the same rate law. C) the math involved is too complicated. D) all of the above. 1 4 Question 11 Which of the following can be used to determine the rate of a chemical reaction? 20% 20% 20% 2 3 20% 20% A) Measuring a change in color B) Measuring a change in mass C) Measuring a change in concentration of a certain species D) All of the above E) None of the above 1 4 5 Question 12 The average rate of a reaction is the rate of reaction at any given time. A) True B) False 50% 50% 1 2 Question 13 The rate of a reaction 25% A) is always dependent of the concentration of the reactants. B) may or may not depend on reactant concentration. C) is never constant throughout a reaction. D) can be calculated for first order and second order reactions only. 1 25% 25% 2 3 25% 4 Question 15 In the reaction N2 + 3H2 → 2NH3, the rate of formation of ammonia at a given moment is 0.060 mol/s. What is the rate of reaction of hydrogen gas at the same 20% 20% 20% 20% 20% moment? A) -0.060 mol/s B) 0.090 mol/s C) -0.090 mol/s D) 0.040 mol/s E) -0.040 mol/s 1 2 3 4 5 Question 16 The reaction C2H6 → 2CH3· is first order with k = 2.50x10-3 s-1. How much time will be necessary for the concentration of C2H6 to decrease to 20 % of the initial 20% 20% 20% 20% 20% amount? A) 644 s B) 280. s C) 0.0025 s D) 0.0005 s E) None of the above 1 2 3 4 5 Question 17 The reaction C2H6 → 2CH3· is first order with k = 2.5x10-3 s-1. Which of the following changes in concentration will last longer than the others? 20% 20% 20% 2 3 20% 20% A) 2.0 M → 1.0 M B) 1.0 M → 0.50 M C) 0.50 M → 0.25 M D) 0.80 M → 0.40 M E) None of the above 1 4 5 Question 18 Which type of plot will be linear for a second 20% 20% 20% 20% order reaction? 20% A) [A] vs time B) ln[A] vs time C) 1/[A] vs time D) [A]2 vs time E) None is linear 1 2 3 4 5 Question 20 A unimolecular reaction follows first order kinetics, and a bimolecular reaction follows second order kinetics. A) True B) False 50% 50% 1 2 Answer Key – Chapter 13 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. D A E D D B B B B A 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. D B B C C A A C C B