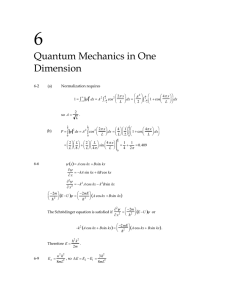
( ) ( ) x
advertisement
6-6
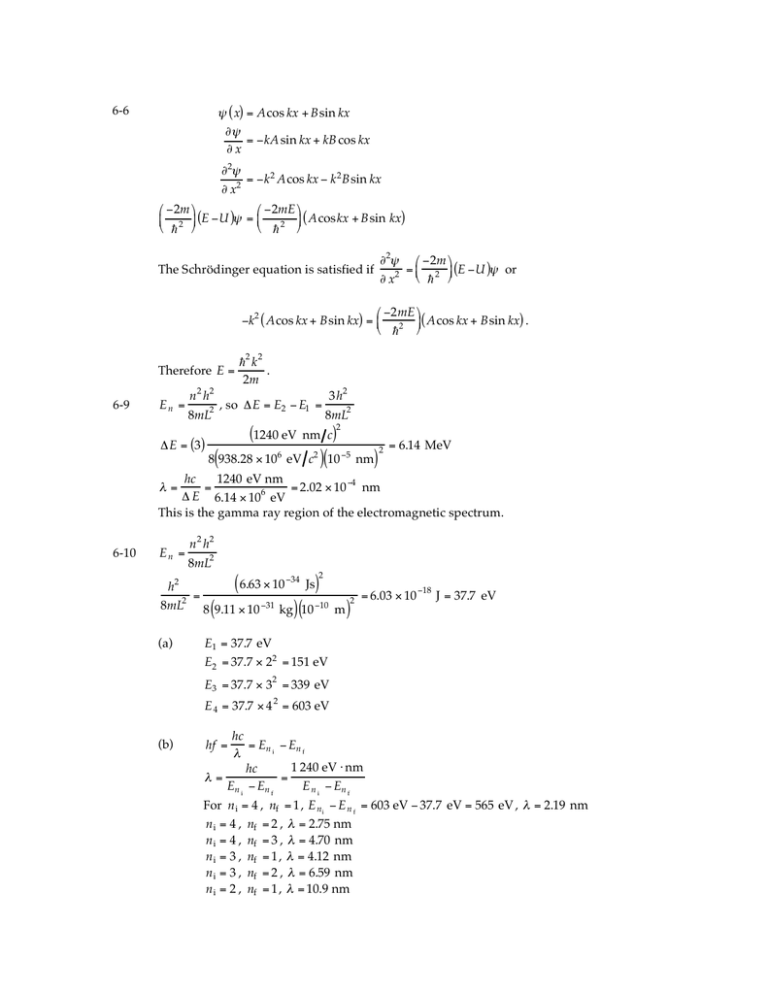
" ( x) = Acos kx + Bsin kx
#"
= $kA sin kx + kB cos kx
#x
#2"
= $k 2 Acos kx $ k 2 Bsin kx
# x2
% $2m (
% $2mE (
' 2 * (E $U )" = '
* ( Acoskx + Bsin kx)
& h )
& h2 )
The Schrödinger equation is satisfied if
"2# % $2m (
='
* (E $U )# or
" x2 & h 2 )
# "2mE &
"k2 ( Acos kx + Bsin kx) = % 2 (( Acos kx + Bsin kx) .
$ h '
Therefore E =
6-9
En =
h2 k 2
.
2m
n 2 h2
3h2
,
so
"E
=
E
#
E
=
2
1
8mL2
8mL2
"E = (3)
(1240 eV nm c)2
(
)(
)
8 938.28 # 106 eV c2 10 $5 nm
2
= 6.14 MeV
hc
1240 eV nm
=
= 2.02 $ 10 %4 nm
# E 6.14 $ 106 eV
This is the gamma ray region of the electromagnetic spectrum.
"=
2 2
6-10
En =
n h
8mL2
2
(
)
6.63 " 10 #34 Js
h2
=
8mL2 8 9.11 " 10 #31 kg 10 #10 m
(
(a)
)(
2
)
= 6.03 " 10 #18 J = 37.7 eV
E1 = 37.7 eV
E2 = 37.7 " 22 = 151 eV
E3 = 37.7 " 32 = 339 eV
E 4 = 37.7 " 4 2 = 603 eV
(b)
hc
= En i # En f
"
1 240 eV $ nm
hc
"=
=
En i # En f
E n i # En f
hf =
For n i = 4 , nf = 1 , E ni " E n f = 603 eV " 37.7 eV = 565 eV , " = 2.19 nm
n i = 4 , nf = 2 , " = 2.75 nm
n i = 4 , nf = 3 , " = 4.70 nm
n i = 3 , nf = 1 , " = 4.12 nm
n i = 3 , nf = 2 , " = 6.59 nm
n i = 2 , nf = 1 , " = 10.9 nm
6-12
#( 3 8 ) h" &
hc $ h2 ' 2 2
(
"E =
=&
and L = %
2 ) 2 *1
# % 8mL (
%$ mc ('
6-13
(a)
[
12
]
= 7.93 ) 10 *10 m = 7.93 Å.
Proton in a box of width L = 0.200 nm = 2 " 10
#10
m
2
(
)
6.626 " 10 #34 J $ s
h2
E1 =
=
8mp L2 8 1.67 " 10 #27 kg 2 " 10 #10 m
(
)(
)
2
= 8.22 " 10 #22 J
#22
=
(b)
8.22 " 10
J
= 5.13 " 10 #3 eV
#19
1.60 " 10
J eV
Electron in the same box:
2
(
)
6.626 " 10 #34 J $s
h2
E1 =
=
8me L2 8 9.11 " 10 #31 kg 2 " 10 #10 m
(
6-14
)(
= 1.506 " 10 #18 J = 9.40 eV .
2
)
(c)
The electron has a much higher energy because it is much less massive.
(a)
Still,
n"
h nh
= L so p = =
2
" 2L
2%
"
K = $c2 p2 + mc2 '
#
&
(
)
12
") nhc , 2
2
E n = $+
. + mc
$#* 2L -
( )
") nhc , 2
2
K n = $+
. + mc
$#* 2L -
(
(b)
"12
m , m = 9.11 " 10
Taking L = 10
The nonrelativistic result is
#31
( )
( mc 2 = E ( mc 2
1 2
2%
'
'&
2%
,
12
) ''
( mc2
&
kg , and n = 1 we find K1 = 4.69 " 10 #14 J .
(
)
2
6.63 " 10 #34 J $ s
h2
E1 =
=
= 6.03 " 10 #14 J
8mL2 8 9.11 " 10 #31 kg 10 #24 m 2
(
)(
)
Comparing this with K1 , we see that this value is too big by 29%.
6-16
(a)
$ # x'
" ( x) = Asin & ) , L = 3 Å. Normalization requires
% L(
L
L
% $ x(
LA2
2
1 = # " dx = # A2 sin2 '
dx
=
*
& L )
2
0
0
" 2%
so A = $ '
# L&
12
L3
P=
(b)
$2'
L3
$ * x'
2
* 3
( 3)1 2 0
2 -*
2
2
2
# " dx = &% L )( # sin &% L )( dx = * # sin +d+ = * // 6 , 8 22 = 0.195 5 .
0
0
0
.
1
12
$ 100# x '
" 2%
" = Asin&
,
A
=
)
$# '&
% L (
L
P=
L3
100" 3
# 100 " x&
2
2# L &
1 , 100" 1
# 200" & /
2
sin2 %
+ sin%
)
( dx = %$
(' ) sin *d* =
.
$ 3 (' 10
L 0
$ L '
L 100"
50" - 6
4
0
1 , 1 / # 2" & 1
3
+.
sin%
= 0.331 9
(' = +
1
$
3 - 200" 0
3
3 400"
Since the wavefunction for a particle in a one-dimension box of width L is given by
$ n# x '
2
2
2 $ n# x'
" n = Asin&
) it follows that the probability density is P( x) = " n = A sin &
),
% L (
% L (
which is sketched below:
=
6-18
P(x)
"
2
"
3"
2
5"
2
2"
From this sketch we see that P( x) is a maximum when
n" x
L
3"
n" x
L
=
" 3" 5"
#
1&
,
,
, K = "% m + (
$
2 2
2
2'
or when
x=
L"
1%
$# m + '&
n
2
Likewise, P( x) is a minimum when
x=
!
6-24
Lm
n
m = 0, 1, 2, 3, K, n .
n" x
= 0 , " , 2" , 3" , K = m" or when
L
m = 0, 1, 2, 3, K, n
After rearrangement, the Schrödinger equation is
U( x) =
d2" # 2m &
=%
( {U (x) ) E}" (x) with
dx2 $ h 2 '
1
#$ x 2
gives
m" 2 x2 for the quantum oscillator. Differentiating " ( x) = Cxe
2
2
d"
= #2$ x" ( x) + C #$ x
dx
and
!
!
2$ xd"
2
d2"
2
# 2$" ( x) # (2$ x)Ce #$ x = ( 2$ x) " ( x) # 6$ " (x) .
2 =#
dx
dx
Therefore, for " ( x) to be a solution requires
(2" x)2 # 6" = 2m2 {U( x) # E} = %'& mh$ (*)
2
2 mE
. Equating coefficients of like terms gives
h
h2
3" h2 3
2 mE
m#
m#
and 6" = 2 . Thus, " =
and E =
= h# . The normalization
2" =
m
2
h
2h
h
$
integral is 1 =
x2 #
2
2
2
2 #2& x
dx where the second step follows from the
% " ( x) dx = 2C % x e
#$
symmetry of the integrand about x = 0 . Identifying a with 2" in the integral of Problem
1 &# ) &
6-32 gives 1 = 2C % ( %
$ 8" ' $ 2" ('
2#
6-25
12
$ 32" 3 '
or C = &
)
% # (
1 4
.
At its limits of vibration x = ±A the classical oscillator has all its energy in potential form:
# 2E &
1
E = m" 2 A2 or A = %
$ m" 2 ('
2
12
"
1%
. If the energy is quantized as E n = $ n + ' h( , then the
#
2&
#( 2n + 1)h &
corresponding amplitudes are An = %
(
$ m" '
6-29
(a)
12
.
Normalization requires
$
$
2
$
2
(
)
(
)
1 = % " dx = C 2 % e #2x 1 # e #x dx = C 2 % e #2x # 2e #3x + e #4x dx . The integrals are
0
#$
0
# 1 & 1 , C2
2 )1
elementary and give 1 = C * " 2% ( + - =
. The proper units for C are those
$ 3 ' 4 . 12
+2
"1 2
of (length )
(b)
!
12
thus, normalization requires C = (12)
nm "1 2 .
The most likely place for the electron is where the probability "
2
is largest. This
d"
is also where " itself is largest, and is found by setting the derivative
equal
dx
zero:
0=
!
d"
= C #e #x + 2e #2x = Ce #x 2 e #x # 1 .
dx
{
}
{
}
"x
The RHS vanishes when x = " (a minimum), and when 2e = 1 , or x = ln 2 nm .
Thus, the most likely position is at xp = ln 2 nm = 0.693 nm .
(c)
!
The average position is calculated from
$
#$
!
2
$
(
)
2
$
(
)
x = % x" dx = C 2 % xe #2x 1 # e #x dx = C 2 % x e #2x # 2e #3x + e #4x dx .
0
0
#
The integrals are readily evaluated with the help of the formula
1
"ax
$ xe dx = a 2 to
0
)1 # 1& 1 ,
2
"1
2 ) 13 ,
get x = C * " 2% ( + - = C *
- . Substituting C = 12 nm gives
$
'
4
9
16
144
+
.
+
.
2
x =
13
nm = 1.083 nm .
12
We see that x is somewhat greater than the most probable position, since the
probability density is skewed in such a way that values of x larger than xp are
weighted more heavily in the calculation of the average.
6-31
2
The symmetry of " ( x) about x = 0 can be exploited effectively in the calculation of
average values. To find x
$
2
x = % x" (x) dx
#$
We notice that the integrand is antisymmetric about x = 0 due to the extra factor of x (an
odd function). Thus, the contribution from the two half-axes x > 0 and x < 0 cancel
2
exactly, leaving x = 0 . For the calculation of x
, however, the integrand is symmetric
and the half-axes contribute equally to the value of the integral, giving
#
#
2
x = $ x2 " dx = 2C 2 $ x2 e %2x
0
x0
dx .
0
!
3
" x0 %
Two integrations by parts show the value of the integral to be 2$ ' . Upon substituting
# 2 &
!
12
$ x2 '
" 1 % " x0 % 3 x20
x
2 12
2
= & 0 ) = 0 . In
for C , we get x = 2 $ ' (2 )$ ' =
and "x = x # x
# x0 & # 2 &
2
% 2(
2
calculating the probability for the interval "#x to +"x we appeal to symmetry once
again to write
2
(
2
!
+$x
2
2
$x
P = % " dx = 2C % e
#2x x 0
0
#$x
)
x )
dx = #2C ( 0 +e #2x
' 2*
2&
$x
x0
= 1 # e#
2
= 0.757
0
or about 75.7% independent of x0 .
6-32
2
2 #ax 2
The probability density for this case is " 0 (x) = C0 e
$
For the calculation of the average position x =
#a&
with C 0 = % (
$" '
1 4
and a =
m"
.
h
2
% x" 0 ( x) dx we note that the integrand
#$
!
!
!
!is an odd function, so that the integral over the negative half-axis x < 0 exactly cancels
2
that over the positive half-axis ( x > 0 ), leaving x = 0 . For the calculation of x ,
2
however, the integrand x " 0
giving
!
2
is symmetric, and the two half-axes contribute equally,
2
x
= 2 C02
#
2 "ax 2
$x e
dx
1 (% + (
& 4 a *)'& a *)
%
= 2C 20 '
0
.
12
(a)
% h (
1
h
2 12
2
and "x = x # x
.
='
=
& 2 m$ *)
2 a 2m"
Since there is no preference for motion in the leftward sense vs. the rightward
sense, a particle would spend equal time moving left as moving right, suggesting
px = 0 .
(b)
To find px
(
Substituting for C 0 and a gives x2 =
6-33
12
2
)
we express the average energy as the sum of its kinetic and
px2
px2
+ U =
+ U . But energy is sharp
potential energy contributions: E =
2m
2m
1
in the oscillator ground state, so that E = E0 = h" . Furthermore, remembering
2
1
h
2 2
that U( x) = m" x for the quantum oscillator, and using x2 =
from
2
2 m"
1
1
Problem 6-32, gives U = m" 2 x2 = h" . Then
2
4
$
h
#
'
mh
#
.
p2x = 2m( E0 " U ) = 2m&
=
% 4 )(
2
(c)
6-34
(
2
"px = px # px
2 12
)
% mh$ (
='
& 2 *)
12
$ h '
From Problems 6-32 and 6-33, we have "x = &
% 2 m# )(
12
12
12
$ mh# '
and "px = &
% 2 )(
12
. Thus,
$ h ' $ mh# '
h
"x"px = &
= for the oscillator ground state. This is the minimum
% 2m# )( &% 2 )(
2
uncertainty product permitted by the uncertainty principle, and is realized only for the
ground state of the quantum oscillator.
!
!
!
!