PHARMACOKINETICS OF LOCAL GROWTH FACTOR DELIVERY
IN MYOCARDIAL TISSUE
SArnFp
By
W,
-fot
IWIMWN
Kha N. Le
B.S., Bioengineering
University of California, San Diego 1998
2002
JUL 3 1
LIBRARIE2
SUBMITTED TO THE DEPARMENT OF ELECTRICAL ENGINEERING AND
COMPUTER SCIENCE IN PARTIAL FULFILLMENT OF THE REQUIREMENTS
FOR THE DEGREE OF
MASTER OF SCIENCE IN ELECTRICAL ENGINEERING AND COMPUTER SCIENCE AT THE
MASSACHUSETTS INSTITUTE OF TECHNOLOGY
JUNE 2002
@ 2002 Kha N. Le. All rights reserved
The author hereby grants to MIT permission to reproduce and to distribute publicly paper
and electronic copies of this +hzceiq (icument in whole or in part.
Signature of Author:
Department of Electrical Engineering and Computer Science
May 10, 2002
Certified by:.
('I
Accepted by:
Elazer R. Edelman
Thomas D. and Virginia W. Cabot Professor
Division of Health Sciences and Technology
Thesis Supervisor
A'thur C. Smith
Science
Computer
and
Professor of Electrical Engineering
Chair, Committee on Graduate Students
of Electrical Engineering and Computer Science
PHARMACOKINETICS OF LOCAL GROWTH FACTOR DELIVERY IN MYOCARDIAL TISSUE
By
Kha N. Le
Submitted to the Department of Electrical Engineering and Computer Science on May 10, 2002 in
Partial Fulfillment of the Requirements for the Degree of Master of Science in Electrical
Engineering and Computer Science
ABSTRACT
An emerging approach for the treatment of ischemic heart disease is the induction of
angiogenesis by means of the locally delivering growth factors to the myocardium. When
deposited within heart tissue the compounds elicit a vascular response that is hoped to perfuse
ischemic myocardium. There is, however, little quantitative data on macromolecular transport in
myocardium, their fate after being delivered, how their transport is affected by structural properties
of myocardial tissue, and in-vivo conditions such as the convection of blood in the highly vascular
capillary network. Attempts to find effective ways of delivering therapeutic macromolecules to
myocardium that could maximize the impact of the agents and minimize systemic toxicity and
adverse side effects have been hampered by the minimal understanding of transport in the
complex myocardial tissue under varying in-vivo conditions. This thesis investigates
macromolecular transport mechanism in the myocardium by examining the role of diffusion,
equilibrium average tissue binding, and capillary convection.
Epidermal growth factor (EGF) and basic fibroblast growth factor (FGF-2) were chosen as
model growth factor because of their potency of inducing endothelial mitosis and angiogenesis invitro. The "effective" diffusivity and partition coefficient of radiolabeled EGF and FGF-2 in rat
myocardium were obtained with a diffusion cell in minimal time assuring tissue integrity and
protein stability. A three-dimensional continuum pharmacokinetic model that takes into account
realistic coronary capillary network configuration and morphometry was constructed to simulate
transport of generic macromolecules in a highly vascular tissue such as the myocardium. Partition
coefficients of EGF and FGF-2 were 0.26 and 1.34, and diffusivities 1.42 and 4.58 lim 2/s,
respectively. The impact of vasculature was evaluated in a computational model constructed
based on these findings. At steady state equilibrium, total drug deposition and penetration depth
of macromolecules in physiologic range in myocardium were shown to be much less than that for
solid tissue that is not perfused by capillary network. Drug transport varied inversely as functions
of intimal permeability and capillary density. Results from this study provided insights into the
design of myocardial drug delivery systems, and drug engineering with a hope to better
angiogenic treatment for ischemic heart disease.
Thesis Supervisor: Elazer R. Edelman
Title: Thomas D. and Virginia W. Cabot Professor,
Division of Health Sciences and Technology.
2
ACKNOWLEDGMENTS
First of all, I would like to express my appreciation to my advisor, Professor Elazer
Edelman, for allowing me to join the lab, and directing me to this great new field. He has been a
wonderful advisor who is always available for advice despite his busy schedule. I am grateful for
his intellectual guidance and penetrating insights. His great scientific and clinical knowledge,
patient, and understanding have guided me throughout my years in the lab and made my research
experience a meaningful and enjoyable one. He is an extraordinary role model for both my
professional and personal development. I consider myself extremely lucky for having Elazer as my
thesis advisor.
I would also like to thank all members of the Edelman lab who helped me settle into a more
than academic and scientific environment. My special thanks go to Chao-Wei Hwang for many
insightful discussions, his mathematical and programming skills and persistence to find solutions
to my problems, David Ettenson for his great biology and general knowledge, and patient to my
relentless questions, Aaron Baker, Wen-hua Fan, Kumaran Kolandaivelu and David Wu for their
ideas and technical helps, Philip Seifert for his expertise in histology and helps, and Pam Li and
Geeta Nagpal for assisting this research. I am grateful to Elazer, Chao-Wei, and David Ettenson for
the many hours refining my humble English and improvement of this thesis.
I also owe great gratitude to my undergraduate research advisors, Drs. Ghassan Kassab and
Y.C. Fung, for their excellent mentorship and helping me to get to where I am today.
I would like to thank my family, my parents and sister, for their unconditional caring love
and support, and their strong values of family, morality and emphasis in the importance of higher
education. Most importantly, I dedicate this thesis to my wife and best friend, Thoa, whose love,
understanding, comfort, and encouragement make everything meaningful.
3
TABLE OF CONTENTS
TA B LE O F C O N T E N TS .........................................................................................................................
L IS T O F F IG U R E S ....................................................................................................................................
IN T R O D U C T IO N ................................................................................................
C HA P T E R 1
4
6
. 7
7
1.1 O bjective ....................................................................................................................................................................
1.2 Thesis Organization..............................................................................................................................................7
C HA P T E R 2
9
B AC K G R O U N D ........................................................................................................
9
2.1 M otivation..................................................................................................................................................................
2.2 Induction of Collateral Circulation as a Treatment of Ischemic Heart Disease.................9
9
2.2.1 Ischem ic Heart Disease ..................................................................................................
....
12
.......................................
Disease
2.2.2 Current Treatments of Ischemic Heart
.13
2.2.3 Collateral Circulation of the Heart .................................................................................
16
2.2.4 Angiogenesis Growth Factor Delivery as a Treatment for IHD ...................................
17
2.3 The need to understand growth factor transport in myocardium ..........................................
2.4 C ontinuum P harm acokinetics.......................................................................................................................18
QUANTIFICATION OF EGF AND FGF-2 DIFFUSION
CHAPTER 3
C O E FFIC IE N T IN M Y O C AR D IU M .............................................................................................
.
22
22
3.1 Introduction ............................................................................................................................................................
3.2 M aterials and M ethods......................................................................................................................................24
3.2.1 lodination of EGF and FGF-2.................................................................................................................................24
3.2.2 Tissue Preparation and Measurem ent of Partitoning .......................................................................................
26
3.2.3 Measurem ent of Effective Diffusivity.....................................................................................................................
26
3.2.4 SDS-PAG E Assay for EGF and FGF-2 Integrity ..............................................................................................
29
3.3 R esults ......................................................................................................................................................................
3 .3 .1 Pa rtitio n C o e ffi c ie n t ................................................................................................................................................
3 .3 .2 Effe ctiv e D iff u s ivity .................................................................................................................................................
31
31
34
3.4 D iscussion...............................................................................................................................................................38
3.4.1 Diffusivity M easurem ents in Vascularized Tissue ...........................................................................................
38
3.4.2 EGF and FGF-2 Partition Coefficients and Diffusivities.....................................................................................
39
CHAPTER 4 COMPUTATIONAL MODELING OF MACROMOLECULAR
TRANSPORT IN VASCULARIZED TISSUE ........................................................................
41
4.1 Introduction ............................................................................................................................................................
41
4.1.1 Macrom olecular Transport in Vascularized versus Solid Tissue.......................................................................
41
4 .1 .2 Tra n s p o rt M e c h a n is m s...........................................................................................................................................
42
4 .1 .2 .1 D iffu sio n ..........................................................................................................................................................
42
4 .1 .2 .2 C o n vectio n ......................................................................................................................................................
45
4 .1 .2 .3 P e rm e a tio n .....................................................................................................................................................
45
4.1.3 Capillary Network in Myocardium ..........................................................................................................................
46
4.1 M aterials and M ethods......................................................................................................................................
47
4
4.2.1 Transport Processes in Cardiac Tissue:.................................................................
47
4.2.2 Capillary Network G eneration .................................................................................................
49
4.2.3 Num erical M ethods .....................................................................................................
.................... 51
4.2.4 Boundary Conditions and Initial Conditions...............................................................................
..........................................................................................................................................
4.2.5 Assum ptions
4.3 Results ...............................................................................................................................
4.3.1 Capillaries act as sinks for transport ...............................................................................
4.3.2 Myocardial Transport Models..........................................................................................
.............
55
56
.................. 56
.................
4.3.2.1 Spatial Distribution .........................................................................................................................
. .....................
4.3.2.2 Total Tissue Deposition............................................................................................
4 .3 .2 .3 C a p illa ry D e ns ity ......................................................................................................
53
. . .....
60
60
62
................. 64
4.4 D iscussion..............................................................................................................................................................66
4.4.1 Vascularized Tissue Drug Delivery........................................................................................................................
66
4 .4 .2 Im p licatio n s .............................................................................................................................................................
67
4.4.2.1 Norm al and
Ischem ic Myocardial Drug Transport: ....................................................................................
67
4.4.2.2 Controlled Release Device Engineering:..................................................................................................
68
4 .4 .2 .3 D ru g En g in e e rin g ...........................................................................................................................................
68
4 .4 .2 .4 D ru g Hy d ro p h o bic ity .......................................................................................................................................
69
4.4.2.5 Tem poral Evolution of Drug Deposition and Distribution............................................................................
69
C HAP TE R 5
C O N C LU S IO N .........................................................................................................
5.1 A cco m plishm ents ................................................................................................................................................
71
71
5.2 Future Work.............................................................................................................................................................71
C HA PT E R 6
A P P E N D IC E S ..........................................................................................................
73
6.1 Partition C oefficient and D iffusivity D ata...............................................................................................
73
6.1.1 Partition Coeffficient Data..................................................................................................................
73
6 .1 .2 D iffu s ivity D ata ........................................................................................................................................................
75
6.2 Matlab Code for Simulations of Myocardial Drug Transport ......................................................
77
B IB LIO G R A P H Y .......................................................................................................................................
91
5
LIST OF FIGURES
FIGURE 1: NORMAL AND ATHEROSCLEROTIC ARTERY................................................
11
FIGURE 2: TYPES OF BLOOD VESSEL GROWTH ...........................................................
15
FIGURE 3: CAPILLARY NETWORK .................................................................................
20
FIGURE 4 IOD IN ATION PROFILE .....................................................................................
25
FIGU RE 5: D IFFUSIO N CELL ............................................................................................
27
FIGURE 6: SEMI-INFINITE SOLUTION.............................................................................
27
FIGURE 7: ILLUSTRATION OF THE COMPLIMENTARY ERROR FUNCTION. ............
28
FIGURE 8: TIME TO EQUILIBRIUM.................................................................................
32
FIGURE 9: PARTITION COEFFICIENT .............................................................................
33
FIGURE 10: EFFECTIVE DIFFUSIVITY.............................................................................
35
FIGU RE 11: SD S-PA GE RE SU LTS......................................................................................
37
FIGURE 12: GENERATED CAPILLARY CONFIGURATION............................................
50
FIGURE 13: BOUNDARY CONDITION.............................................................................
54
FIGURE 14A: 2D CAPILLARY SIMULATION: CAPILLARY AS SINK: X-PROFILE .....
58
FIGURE 14B: 2D CAPILLARY SIMULATION: CAPILLARY AS SINK: Y-PROFILE .....
59
FIGURE 15: SPATIAL DISTRIBUTION ............................................................................
61
FIGURE 16: TO TA L D EPO SITION ......................................................................................
63
FIGU RE 17: CAPILLARY DEN SITY .................................................................................
65
FIGURE 18: TOTAL DEPOSITION AS FUNCTION OF CAPILLARY DENSITY..............
65
6
CHAPTER 1
INTRODUCTION
1.1 Objective
Angiogenic growth factors are increasingly introduced into heart tissue to evoke specific
vascular responses, and yet the transport mechanism of these factors in myocardial tissue remains
poorly understood.
Heart tissue is composed of complicated three dimensionally orientated
myocytes intertwined in a dense network of capillaries, and defines a unique environment for local
macromolecular transport. This study examines the role of diffusion, capillary convection and
equilibrium average tissue binding in macromolecular transport in myocardial tissue. The objective
of this study is to predict the myocardial diffusivity of angiogenic growth factors, and model their
transport in vascularized tissue. Basic fibroblast growth factor (FGF-2) and epidermal growth
factor (EGF) were used as model molecules to demonstrate the validity of the myocardial growth
factor diffusivity determination method.
1.2 Thesis Organization
This thesis empirically characterizes and mathematically models transport of growth
factors in myocardium. Chapter 2 provides the background and motivation for understanding
macromolecular transport in myocardium. Chapter 3 describes a novel method to obtain growth
factor diffusivity in myocardium. The validity of the method is demonstrated using basic fibroblast
growth factor (FGF-2) and epidermal growth factor (EGF) in excised rat hearts. This chapter also
defines the average myocardial binding property of the two growth factors by determining their
partition coefficients in tissue. Chapter 4 uses computational modeling to propose a perspective for
the role of coronary capillary convection in myocardial macromolecular drug transport and
7
discusses its implications in drug delivery to vascularized tissue. Chapter 5 summarizes the thesis
and suggests relevant future work.
8
CHAPTER2
BACKGROUND
2.1 Motivation
Ischemic tissue is poorly perfused and it has long been hoped that direct injection of
angiogenic compounds (like growth factors) could stimulate angiogenesis. Such an approach might
offer promise for patients with diffuse coronary and peripheral artery disease, absent conduits after
previous bypass operations, small distal vessels or for those who cannot undergo standard
revascularization procedures. Many angiogenic agents have been directed to myocardial tissue to
induce new vessels or enhance collateral circulation. Yet, while different modes of local
administration e.g. intrapericardial or intramyocardial, have been undertaken in both animal
research and clinical settings, much is unknown about the optimal delivery and actual clinical
effect of these potential angiogenic factors. Delivering drug without knowledge of its fate can do
more harm than good. For instance, since angiogenic growth factors are potent smooth muscle
mitogens, their delivery in the vicinity of vascular plaques might exacerbate neointima thickening.
Understanding of drug transport in myocardium will provide a framework for evaluating safe and
effective drug delivery systems and monitoring clinical trials and use.
2.2 Induction of Collateral Circulation as a Treatment of Ischemic Heart
Disease
2.2.1 Ischemic Heart Disease
Ischemic heart disease (IHD) affects 12.6 million Americans and remains the leading cause
of mortality and morbidity, accounting for 1/5 of U.S. deaths in 1999[1]. Ischemia is the byproduct
of inadequate supply of oxygen and nutrients to a tissue. Atherosclerotic in particular creates
9
thickening of the arterial wall, loss of arterial elasticity and obstruction of the vascular lumen
reducing flow. Figure 1 demonstrates the histologic features of a typical atheromatous plaque next
to a healthy normal artery. The characteristic processes of atherosclerosis are intimal hyperplasia
and lipid accumulation. Although myocardial ischemia often results from arteriosclerosis of the
major coronary arteries, it can also occur in the smaller arteries and arterioles most often associated
with hypertension and diabetes mellitus. In diffuse smaller coronary artery disease, the two
anatomic variants, hyaline and hyperplastic, cause thickening of vessel walls with luminal
narrowing that may induce downstream ischemic injury[2].
10
FIGURE 1: Normal (1A) and atherosclerotic (1B) arteries. Structures depicted include
internal elastic lamina (A), media (B), external elastic lamina (C), and the typical
components associated with athersclerotic plaque: fibrous cap, lipid and calcium rich
regions (D). Notice the marked reduction in size of the arterial lumen (E).
11
2.2.2 Current Treatments of Ischemic Heart Disease
Contemporary treatments for IHD include pharmacologic and mechanical approaches.
Potent thrombolytic drugs can dissolve clots but leave the atherosclerotic lesion intact. Balloon
angioplasty and endovascular stents displace vascular lession, and graft surgery bypasses them. All
three of these procedures have high initial success rate but the potential for abrupt reclosure and the
development of proliferative restenosis requires re-intervention within 4-6 months after the
procedure[2].
Specifically, balloon angioplasty is an interventional procedure wherein the stenosed artery
is dilated by a percutaneous insertion of a balloon-tip catheter into the artery. Increasingly common
in these procedures, endovascular stents are also used. Stents are expandable metal mesh tubes
made from a variety of materials including stainless steel, titanium and nitinol. Prior to catheter
insertion, a stent is mounted on a balloon catheter tip in a compressed state and is expanded when
at the site of stenosis after threaded through the vascular tree. Due to their configuration and
material property, the stent stays open, holding the narrow artery patent, and is left as a permanent
implant within the artery. Although short term results seem promising, about 1/3 of stented patients
require further intervention within six months[3] to restore vessel lumen after thrombosis, fibrosis
and proliferating neointimal hyperplasia.
Regularly when these minimally invasive surgical
techniques have been exhaustively attempted and unsuccessfully at keeping the artery
unobstructed, patients undergo coronary artery bypass graft surgery, where grafts of either
autologous saphenous vein or internal mammary artery are surgically put in as conduits bypassing
the occluded artery. Even though most patients do well for extended periods after surgery, many
develop late recurrence of symptoms because of either graft occlusion or progression of
atherosclerosis in the native coronary distal to the grafts[2].
12
It is becoming readily apparent that practically any mechanical intervention designed to
manage atherosclerotic arteries often inflict damage to the very tissues they were intended to help,
resulting in an accelerated atherosclerosis of its own[4]. The factors causing restenosis are
complex. The mechanical processes of balloon dilation, stent expansion, and surgical bypass
impose injurious stimuli that reduce endothelial and/or smooth muscle integrity, and leads to
infiltration of monocytes and macrophages, and aberrant vasospasm. Restenosis ensues with the
subsequent migration and proliferation of medial smooth muscle cells to form a neointima[5-7].
This accumulation of cells can be so massive as to obstruct the arterial lumen and threaten organ
integrity, reversing any benefit from the original intervention.
2.2.3 Collateral Circulation of the Heart
The problems associated with mechanical interventions have generated interest in
salvaging myocardium through the induction of collateral blood vessel formation using angiogenic
growth factors. The presence of coronary collateral circulation has been defined in patients whose
coronary occlusions are discovered coincidentally after death from non-cardiac causes or patients
with symptomatic ischemic heart disease who may have had extensive occlusions but an intact
myocardium[8]. These cases suggested that patients sometimes benefited from a natural and
gradual development of extensive collateral circulations.
New blood vessel development has been described as three distinct types (Figure 2):
vasculogenesis, angiogenesis and arteriogenesis. Vasculogenesis is a developmental process
involving in-situ formation of blood vessels from endothelial progenitor cells in the embryo[9, 10].
Angiogenesis refers to the extension of already formed primitive vasculature by sprouting of new
capillaries through migration and proliferation of previously differentiated endothelial cells.
13
Angiogenesis occurs both in embryonic development[1 1], and in adults in response to tissue
ischemia[12],
and with development of collateral vessels after ex vivo expansion and
transplantation[ 13]. Angiogenesis also occurs in various adaptative processes not associated with
ischemia, including response to exercise training[14], cardiac postnatal growth[15], and thyroid
hormone-induced cardiac hypertrophy[16, 17]. Arteriogenesis is the growth of collateral vessels
with a well-developed tunica media from preexisting arterioles[18]. These new vessels are often
larger in size than capillaries, and as they can deliver more blood necessary to maintain tissue
integrity, they may be a more effective adaptive process to ischemia[19].
14
a
,
Angiogenesis
Vaslogeeee
Vasculogenesis
W
Smooth muscle cell progenitor
0
Smooth muscle cell
t00000p000000
*
Endothelial cell progenitor
o
Endothelial cell
Arteriogenesis
FIGURE 2: Mechanisms of blood vessel growth. Angiogenesis is the sprouting of
capillaries; Vasculogenesis is the in-situ development of large vessels from precursor
cells; and arteriogenesisis the in-situ growth of arteries from pre-existing arteriolar
anastomoses. (reproduced from Schaper 1999)
15
2.2.4 Angiogenesis Growth Factor Delivery as a Treatment for IHD
Given the use of collateral vessel induction as a natural body adaptation and protective
mechanism from ischemia, a logical approach to treatment of IHD might involve administration of
an appropriate stimulus to create and/or enhance the development of collateral vessels. Researchers
have long known that natural angiogenic growth factors are required to stimulate the growth of
new blood vessels. One of the first angiogenic factors, basic fibroblast growth factor (bFGF), was
purified by Michael Klagsbrun and Yuen Shing in 1985[20]. Since then, many growth factors have
been isolated and shown to induce new blood vessel formation. Four major growth factors that
have been associated with angiogenesis are transforming growth factor P-1 (TGF- 1), plateletderived growth factor (PDGF), basic fibroblast growth factor (FGF-2), and vascular endothelial
growth factor (VEGF/VPF)[21]. However, only studies of angiogenesis with FGF-2 and VEGF
result in induction of functionally significant angiogenesis in various animal models of coronary
artery disease[22, 23].
A large number of angiogenesis-stimulating drugs in animal research and clinical settings
are underway to test various routes of delivery, including intravenous, intra-atrial, intracoronary,
pericardial or direct intramyocardial injections. A major concern with growth factor delivery is the
instability of these proteins. Intravenous biological half-lives of PDGF, FGF-2 and TGF-3 for
example, are 2, 3 and 5 minutes, respectively[21], calling into questions the applicability of
intravenous administration for effective angiogenesis[23-26].
In general myocardial drug
deposition and improvement of collateral blood flow is maximal with direct intramyocardial
16
injection, followed by, in order of decreasing effectiveness, pericardial, intracoronary, Swan Ganz
and intravenous administration[25-3 1].
2.3 The need to understand growth factor transport in myocardium
Angiogenic growth factors must be present at the right concentration for long enough time
at the targeted tissue to realize their full biological potential. It has been known that these cytokines
can be potent at minute doses, but also with disparate effects at different concentrations. Since the
same growth factors that promote the endothelial and smooth muscle cell growth necessary for
angiogenesis and arteriogenesis may induce a proliferative response when exposed to
atherosclerotic plaques, a primary concern is to simultaneously maximize the spatial distribution of
drug effect while restrict their distribution to the interested regions. Until the advent of polymerbased controlled release technology, there has been limited ways in which these proteins can be
delivered to the tissue of interest. Local controlled-release drug delivery allows a sustained and
higher local drug concentration at lower systemic toxicity than what can be achieved if delivered
systemically in a bolus fashion. Various agents have thus been incorporated into drug-eluting
polymer coated onto endovascular stents[32-34], polymeric or fibrin sheets[35], perivascular
wraps[36] or microspheres[37].
Local controlled-release drug delivery has shown promising
results for application of angiogenic growth factors to the myocardium[22, 25, 27, 38].
Despite the numerous advances in angiogenic growth factor delivery, virtually all studies
have looked by necessity at macroscopic endpoints such as symptom improvement, coronary
perfusion changes. The demonstration of clinical benefit requires that one prove increased tissue
perfusion and reduced symptoms and/or enhanced tissue function. Thus many trials have focused
on the primary endpoints without regard for tissue deposition. Yet, it is tissue deposition that may
17
well be the primary determinant of effect, and tissue deposition is very much dependent upon the
physicochemical properties of the drug, kinetics of delivery, binding, and transport processes such
as diffusion, convection, and drug partitioning in tissue[39-41]. Intensive research on arterial drug
transport, both theoretical and experimental, have implicated these mechanisms as critical in
determining spatial tissue drug distribution[39-44].
Although the structural basis for transport[45] and role of physiological forces in local drug
delivery to arterial tissue has been rigorously studied in arterial tissue[39-44], little is known for
myocardial tissue. The factors that determine drug deposition in the heart are more complicated
than that for arterial tissue. Cardiac myocytes are arranged in intricate three-dimensional
configurations perfused by an extensive network of capillaries. Besides the complicated nature of
the static structural arrangement of myocardium, the fact that a large amount of blood flows
through it contributes to the unique local transport environment of the drug. Therefore, it is
necessary to understand how drug transport is affected by these additional factors governing
2.4 Continuum Pharmacokinetics
Compartmental models of pharmacokinetics, where target tissue, organ or organism are
divided or lumped into discrete homogenous compartments, are not sufficient to characterize the
local pharmacological actions in controlled-release local drug delivery. Such models are useful in
describing total or average tissue drug content but fail to take into account of effects of local
structural tissue elements that are potentially crucial in determining spatial tissue drug distribution.
One approach to analysis of local controlled-release delivery is to consider target tissue as a
continuum, where the tissue is divided into infinitesimal elements in which drug molecules
18
distribute based on known physical laws.
Because the computational elements are small,
continuum pharmacokinetics allows the incorporation of local differential anatomical and
structural entities into the model. This is crucial especially in the case of myocardial tissues where
the capillary density is high and the tissue is remarkably heterogeneous.
Furthermore, this
approach enables consideration of local concentration gradients, which may be important because
drugs can be at toxic dose in one region and below therapeutic dose at a nearby tissue region. In
fact, continuum pharmacokinetics has been applied to various tissues: arterial[40, 43, 44],
gastrointestinal tract[46], bronchial tree[47], central nervous system[48], urinary tract[49] and
vaginal[50] to explain and predict experimental data that might escape compartmental models.
The high metabolic demand of the heart requires a rich vascular network and indeed
myocardium consists of myocytes arranged in a complicated three-dimensional configuration and
is perfused by a highly vascular network of 4-6 capillaries per myocyte (Figure 3). This complex
local anatomy is expected to play unique role in dictating local drug transport in heart tissue.
Despite the numerous ongoing angiogenic growth factor delivery studies, no quantitative data exist
to characterize the fate of growth factors in myocardium after delivery. The continuum
pharmacokinetic analysis to understand myocardial growth factor transport provides the rigorous
tools and scientific approach to investigate myocardial drug delivery and offers the hope to bring
angiogenic growth factor delivery to clinical utility.
19
FIGURE 3: Cross section of myocardial tissue showing typical capillary/myocyte
configuration. Myocytes are surrounded by parallel capillaries in direction perpendicular
with the page (white dots), which are connected by distributed cross connection capillaries
(arrows).
20
The lack of knowledge of macromolecular transport in myocardial tissues made it still
unclear how to optimize the delivery of various angiogenic growth factors of different
physicochemical properties to myocardial tissue. This thesis examines the transport of two model
growth factors, FGF-2 and EGF, by specifically considering their diffusive and binding properties
in myocardial tissue. A computational model was also developed to predict the effects of capillary
convection on myocardial transport. Such a quantitative approach to the study of the local
pharmacokinetics and the influence of anatomic factors on the distribution of angiogenic drugs
might shed insight on the biology of myocardial angiogenic response and lead to a more systematic
approach to myocardial drug delivery and drug development.
21
CHAPTER 3
QUANTIFICATION
OF
EGF
AND
FGF-2
DIFFUSION COEFFICIENT IN MYOCARDIUM
3.1 Introduction
Since heart tissue is highly perfused by capillaries, transport of macromolecules in
myocardium can be divided into intravascular and extravascular regions. Convective flow within
the vascular space forces macromolecular transport and distribution. Tissue outside of the blood
vessel consists of many cell types surrounded by relatively complex extracellular matrix, and
transport of macromolecule in this region is often a diffusive process. To understand the nature of
myocardial macromolecular drug delivery, transport in each region needs to be looked at
separately. Fortunately, diffusive transport in the extravascular region can be decoupled from total
transport in in-vitro where there is no capillary convection. In the diffusion-dominated region, as
diffusivity provides a good first order estimate of the fate of drug at a time point after local
delivery, it can be used in-vitro to characterize transport.
Molecular diffusion is governed by Fick's law, where the temporal changes in molecular
concentration is described by the following equation:
ac
at
a 2c
Xax
2
a2 c
8 2c
Y 2
Zaz2
where Dx, Dy and Dz are diffusivities in x, y and z directions, respectively, and c is molecular
concentration. This partial differential equation can be solved to obtain an explicit functional
relationship between molecular concentration with time and/or distance. For a given geometry and
22
boundary condition, the solution can be solved either analytically or computationally. The solution
can then be used to correlate with an experimental spatial or temporal concentration profile to
obtain diffusivity Dx, Dy and D. This task is however not straightforward for diffusion of growth
factor in biological tissues.
Current existing diffusion measurements rely on either 1) spatial distribution[5 1] or 2)
temporal distribution of drug in a diffusion cell setting[52-55]. Methods to determine diffusivity of
macromolecules require thin tissue sections[52-55], long experimental times, or high-resolution,
high sensitivity molecular imaging method[56]. These requirements are incompatible for studying
diffusion of growth factors in living myocardium. For instance, myocardium is highly vascular;
hence methods that require thin membranes of myocardium will increase the likelihood of artifacts
from leakage through medium-to-large diameter vessels. Because of slow macromolecular
diffusion in tissue, standard diffusion cell studies with tissue membranes of thicknesses sufficient
to avoid significant leakage flux would require experimental times greater than 10 hours, during
which times tissue can degrade and most growth factors would be denatured. The measured
diffusivity would be expected to be a gross overestimate of the true diffusivity of intact growth
factors. Wan et. al. proposed a high-resolution fluorescent imaging method for studying transport
of macromolecules in tissue[56]. This method, however, requires a cost-prohibitive mg/mLconcentration of fluorescently-labeled growth factors. With these concerns in mind, we developed
a short-time method of determining diffusivity of growth factors in myocardium.
This chapter quantifies diffusivities of the two model growth factors FGF-2 and EGF using
the short-time method. This measurement provides necessary data to investigate growth factor
transport in vascularized tissues in in-vivo conditions. The concerns of tissue integrity and growth
factor stability are also addressed.
23
3.2 Materials and Methods
3.2.1 lodination of EGF and FGF-2
Exposed tyrosine residues on EGF were labeled with
125I
for myocardial transport
studies[57]. IODO-BEADs (Pierce) were cleaned with 100mM phosphate buffer (pH = 7.5), dried,
and incubated in 100 ul of phosphate buffer. Na12 5I (10 ul, 2mCi, Perkin-Elmer) was then added to
the IODO-BEADs. The reaction mixture was vortexed and incubated for 5 min. Human EGF
(Peprotech, 100 ug in 100 ul phosphate buffer) was then added to the reaction mixture and
incubated for 10 min, determined previously as the optimal duration for the reaction.
FGF-2 was radiolabeled with
1251
using the Bolton-Hunter (BH) reagent (lmCi, Perkin-
Elmer) that targets lysine residues on FGF-2[58]. The BH reagent was dried under a gentle stream
of nitrogen gas. Human recombinant FGF-2 (50ug, Peprotech) was then added to the BH reagent,
and the mixture was incubated on ice for 2.5 hours. To quench the reaction, 200ul of Glycine
(0.2M) was added and incubated on ice for 45 min. 250ul of gel filtration buffer (50mM Tris-HCl,
0.05% gelatin, 1mM dithiothreitol, and 0.3M NaCl, pH=7.5) was added before performing column
chromatography.
Column chromatography (Sephadex G-25) separated labeled EGF and FGF-2 from free 1251
into a series of 0.2 mL aliquots. Figure 4 showed the iodinated protein profile. BioRad Dc protein
assay was performed on the eluent. 20 uL of protein was mixed with 10 uL of Dc Reagent A and
80
uL
of Dc Reagent
B,
incubated
for
15
min
and optical
density
determined
spectrophotometrically at 750nm, to confirm the presence of protein. A standard curve of known
EGF or FGF-2 content quantified the protein products. Eluents high in radioactivity and protein
content were combined to yield the radiolabeled protein stock solution used for later transport
studies.
24
1200000
1000000 800000 -
o
600000 -
E
E
cc
400000 200000 0
1
3
5
7
9
11
13
15
17
19 21
23 25
27 29
Eluted Sample
FIGURE 4: Elution profile after iodination. Each bin corresponds to the radioactivity of 0.2
mL aliquots of column chromatography after iodination. First peak occurring around elutions
numbered 8-10 represents iodinated proteins. Their integrity is verified by SDS-PAGE.
25
3.2.2 Tissue Preparation and Measurement of Partitoning
Adult Sprague-Dawley rats (250-500 g, Charles River Laboratory) were euthanized under
100% CO 2 for 5 min. To measure the partition coefficient of EGF and FGF-2 into myocardium,
the ventricular wall was carefully cut into sections weighing 30-75 mg (wet weight). The sections
were each incubated in 1 mL of
12 5
I-EGF or
125 I-FGF-2
(in KH buffer) at various dilutions (n = 5
for each dilution) for 48 hours at 4 'C to minimize any proteolysis. Pilot studies demonstrated that
drug equilibration for myocardial samples of these sizes occurs in approximately 40 hours. After
equilibrium, the samples were immersed in KH buffer for 2 min to clean off surface adherent
drugs, and the radioactive content was measured using a gamma counter (Crystal Plus, Packard).
The partition coefficient
(K)
was determined as the slope of the linear regression between the
weight-normalized drug content of the tissue sample and the concentration of drug in the
equilibrium incubation baths.
3.2.3 Measurement of Effective Diffusivity
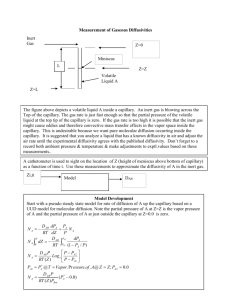
Hearts were cut into 1-2mm thick sections, and mounted between source and sink
compartments of vertical diffusion cells (Figure 5) so that diffusion occurs in transmural direction
(respect to the in-vivo heart).
125
I-EGF or FGF-2 was placed into the source compartment, and
oxygenated Krebs-Henseleit (KH) was placed in sink compartment to maximize the viability of
myocardium. Growth factor tissue deposition was obtained at 30, 120, and 240 minutes from the
radioactive count (gamma counter, Crystal Plus, Packard). As there was no detectable radioactivity
in the sink, no drug diffused all the way through the tissue. In this case, the semi-infinite solution
of the diffusion equation applies.
26
Clamp
SCas
Ulrcut
Myocardium
Sink
FIGURE 5: Vertical diffusion cell. Capacity of source and
sink compartments are 1.5mL and 5mL, respectively.
Myocardium section thickness is approximately 2mm.
L
FIGURE 6: Diffusion solution for semi-infinite media. For
tissue whose thickness is much more than the diffusion front,
i.e. L>>(Dt)A(1/2) where D is diffusivity and t the
experimental time for diffusion to occur, the solution to
diffusion equation can be solved analytically.
27
)[59] exposed to a constant source of drug
For a semi-infinite slab of tissue (L >> v
(Figure 6), the one-dimensional solution to the diffusion equation is
C(x)
-
KCoerfcr
zrDt
where C(x) is the spatial distribution of drug in x direction at a time point t, K is the partitioning
coefficient, Co is the constant drug source, and D is diffusivity. Figure 7 illustrates the curve of
the function
yerfc(x)
2
e _dt=
X
d
efc(x).
1
X
0.9
0.8
One can calculate the
0.7
molecular flux at x=O by differentiation of
x
U
t
the spatial profile. Hence, total drug at a
0.6
0.5
0.4
0.3
time point t becomes
0.2
0.1
M(t) = AD
0
xx :0
KC oetfcr
_
n
10
dt
2 V Di))
10 1
10
x
FIGURE 7: Illustration of the complimentary
error function. x-axis is shown in log-scale.
where M is total accumulated drug in tissue
slab at a time point t, A is the cross sectional area through which drug is exposed to, and D is
diffusivity. After rearranging the terms, the following formula can be obtained
M
-\[
2ACOK
M,0
2ACOic
where M is the total amount of drug deposited in tissue, A is the diffusion cell orifice area, Co is
the source concentration of drug, K is the partition coefficient of drug in tissue which was
determined
28
10
3
in a separate experiment described below, t is the time, and Ms accounts for purely surfaceadherent drug which has not actually diffused into the myocardial tissue. This shows a linear
relationship between the 'scaled mass' quantity
MV
2ACc
and V-1, where the slope of its linear
regression will be Vi5, and it allows us to determine an 'effective' diffusivity can be obtained.
L2
The short time requirement (t << -) of this method to assure that the semi-infinite tissue
D
assumption holds, the likelihood that myocardial tissue properties remain unchanged and growth
factor degradation is minimal is better. Therefore, this 'short-time' method is ideally suited for
studying growth factor diffusion in cardiac tissue.
3.2.4 SDS-PAGE Assay for EGF and FGF-2 Integrity
Since native proteases within the tissue might degrade growth factors and potentially affect
measured diffusivity, physical integrity was confirmed when drug in all diffusion samples was
eluted into 1 mL KH buffer for 2 hours, and the molecular weights of the radioactive protein
content was assessed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDSPAGE)[60, 61]. 10 uL of each drug sample was added to 30 uL of sample buffer (0.95 mL
Laemmli Sample Buffer mixed with 0.05 mL f-Mercaptoethanol, BioRad). This drug mixture was
loaded into the gel (18% Tris-HCl Ready-Made Gel, BioRad) along intact radiolabeled growth
factor, tracking dye (0.1% Edward bromophenol blue, BioRad) and molecular weight standards
(Kaleidoscope, BioRad). The samples were electrophoresed in Tris-Glycine SDS buffer (BioRad)
at 200 V for 30-45 min. The gel was then exposed to a phosphor screen for 6 days and visualized
29
on a phosphorimager (Molecular Dynamics).
Stock
exposed to tissue were also electrophoresed as control.
30
12 5
I-protein samples that have not been
3.3 Results
3.3. 1 Partition Coefficient
Myocardial tissue sections (30-75mg) were incubated in
125I
labeled growth factor (EGF or
FGF-2) until equilibrium to measure the partition coefficient. Although drugs may continue to
exchange between tissue and bulk phase, the time course of FGF-2 tissue concentration showed
that equilibrium is reached within 48 hours (Figure 8). The partition coefficient (K), defined as the
slope of the linear regression between the weight-normalized drug content of the tissue sample and
the concentration of drug in the incubation baths, was determined to be 0.26 and 1.34 for EGF and
FGF, respectively (Figure 9). The affinity of growth factors and their retention to myocardium
heavily depends on the number of tissue specific and non-specific binding sites hence proportional
to the partition coefficient. This more than 5-fold greater affinity of FGF-2 for tissue elements than
EGF arises by virtue of the binding of FGF to fixed heparin or heparan sulfate binding sites in the
extracellular matrix[62].
31
300
-
20)
2250 --
0
00
0 2001000
."
U0
cN50 -o
0
20
40
60
80
Incubation Time (hours)
FIGURE 8: Time to reach steady state equilibrium. At 40 hours of incubation time, tissue
concentration of FGF-2 reaches 99% of steady state equilibrium.
32
. E
r
107
106-
y =1.34x
00
0
o
01
A
R2 =0.97
M 105.
oE.
SE*
104-
103103
104
105
106
107
Bulk Concentration
(Gamma Counts I mL)
0 ft10k
M *108
B
y = 0.26x
R2= 0.98
S 1070
S
106
M
105
104
104
105
106
107
108
1og
Drug Concentration
(Gamma Counts / mL)
FIGURE 9: Partition coefficient of EGF and FGF-2. Since gamma count was determined
to be linearly proportional to drug amount, partition coefficient was defined as slope of
the linear regression line of tissue concentration vs. drug concentration in gammacounts/mK, and determined to be 1.34 and 0.26 for FGF-2 (A) and EGF (B), respectively.
Data shown is Mean ± SE
33
3.3.2 Effective Diffusivity
We defined effective diffusivity as a lumped transport parameter describing the motion of
drug in tissues given an applied concentration gradient and includes, in addition to pure diffusion
from random molecular motion, the effect of steric hindrance within myocardium, nonspecific and
specific binding to tissue elements. This transport parameter gives a first-order estimate of drug
penetration depth at a particular time point after delivery, and facilitates comparison of transport of
different angiogenic growth factors in myocardium.
Tissue deposition of
12'1
radiolabeled EGF or FGF-2 was obtained at 30, 120, and 240
minutes after mounted on the diffusion cell. By measuring the sink drug content, it was determined
that no drug diffused all the way through the tissue. In this case, the semi-infinite solution of the
diffusion equation applies. The results were then fitted to the equation:
2 ACOC
2ACK+
2 ACOIC
(see derivation in 3.2.2)
and diffusivity (D) for '25 1-EGF and "'1-FGF-2 were computed as the square of slope of the linear
regression of scaled mass versus square root of time plot (Figure 10) to be 4.58 and 1.42 um2/sec,
respectively, using partition coefficients of the two compounds determined previously.
34
400
Deff = 1.42 um 2/s
A
3001
y = 1.19x - 7.30
200-
R2= 0.99
0
10004-
20
40
80
60
120
140
120
140
100
Root Time (sec"12)
40G
Deff =4.58 um2 /s
B
E
0t 300
y = 2.14x + 52.8
R2= 0.98
200
-o
10
0
20
40
60
80
100
Root Time (sec" 2)
FIGURE 10: Diffusivity of EGF and FGF-2. Diffusivity was determined as square of the
slope of the linear regression line of scaled mass vs. square root of diffusion time as
derived for short-time method, and determined to be 1.42, and 4.58 um 2 /s for FGF-2 (A)
and EGF (B), respectively. Data shown is Mean ± SE
35
The majority of labeled proteins in the source compartment remain intact as the signal on
the gel located at their molecular weight bands (Figure 11), confirming the validation of our
labeling technique. Furthermore, the labeled proteins eluted from the tissue after 4 hours also
matched the source molecular weight, confirming minimal degradation of EGF and FGF-2 in the
time interval of 'short-time method' diffusion studies.
36
1251.FGF-2
1251-EGF
...................
....................
.......
.....
....................
..........
...................
.......
...
............
...............
................
.
..
........
.......
..........
......
........................
.................
.
..
.................
............ .................
..........................
..................
.....
...................
...............
............
........
................
...
......
.........
................
........
.....
........... . .
..
.........
.............
18kD
7 kD
--------------------........................
................................
...................
M11
...
.............
. ...
.............
...................
.......
.....
..
...
....
..........
....
..
..
..............
................
....................
.
..........
.
.....
.....
.
............
......
.............
....
....
....
........
.......
....................
..
......................
.......................
....................
................
....
........................
..........................
%
....................
...
...
.
..
.
...............
.
.
.
..
.
..
X
X--.................
....
....
.......
.
.....................
...................
.........
.
..........
..........
...........
............
.
............
........................
..........
.
.
..
..
.......................
.
.
..
..
.
I
.
.
..
....
.
..
.
..
.
.
......
...
.
.
.
..
.
..
..
..
...
.............
..........
...................
......
.....
...
....
...............
........................
......
............
.......
.......
.............
.........
......
.
.
.................
...............
....
..
.............
...........................................
...
. . ...
. ..
.......
.........
. .......
. ..........................
FIGURE 11: SDS-PAGE results. For both FGF-2 (A) and EGF (B), the left panels
represent the source and right panels correspond to the eluted proteins from tissue after 4
hours. The two experiments were performed on different type of gel, and different
molecular standards used to indicate the molecular band.
37
3.4 Discussion
3.4.1 Diffusivity Measurements in Vascularized Tissue
The most often methods used to measure molecular diffusivity in biological tissues involve
either the measurement of molecular flux or determination of spatial tissue concentration to fit
using Fick's second law of diffusion[52-55]. As described below, these methods are not
appropriate for measuring molecular diffusivity of growth factor in vascularized tissue including
myocardium. The presence of a rich distribution of small arteries and arterioles in cardiac tissue
introduces low resistance pathways through which molecular flux could be significantly larger than
that of myocardial local environment. In addition, macromolecules with low diffusivity require a
long observation time. This requirement is not compatible for studying of growth factor transport
in myocardium as these factors have a short half-life and the tissue will likely undergo alteration in
structural, chemical and transport properties. For compounds that have low diffusivity hence small
penetration depth, methods involving the spatial molecular distribution measurement require either
a long exposure time to drug source or a relatively high concentration source of fluorescently
labeled growth factors. For the same reasons mentioned previously, we cannot afford to have long
experimental time. Furthermore, at the present time it is cost-prohibitive to work with a large
amount of fluorescently labeled growth factors.
The 'short-time' method proposed in this thesis addresses the complicated issues involved
in determining growth factor diffusivity and transport in myocardium. The minimal time required
to complete this technique assures the integrity of both the growth factors and biological tissue of
interest. SDS-PAGE results (Figure 11) validated the integrity of the growth factors being tracked.
Although there are trace amount of what could be degraded growth factors, the overwhelming
majority of the molar mass at its expected molecular weight. In addition, the popular radioactive
38
growth factor labeling methods used facilitates the data acquisition step since the tissue preparation
involved in this method is minimal, and provides a high sensitive protein quantification method.
The limitations of this short-time method include the need for tissues that are homogenous
or regularly heterogeneous, such as that of myocardium, since the value of diffusivity determined
depends only on the representative tissue portion where drug penetrates. The larger
macromolecules of interest will have shorter tissue penetration depths hence lesser total drug
deposition and lower signal to noise ratio. Furthermore, although the time required for this
diffusivity measurement method is short, it has to be long enough for the signal to noise ratio to be
sufficient for meaningful data. The noise in this case would be the molecules that present on
surface at time t=O, i.e. M, in the equation.
3.4.2 EGF and FGF-2 Partition Coefficients and Diffusivities
The affinity of growth factors and their retention to myocardium heavily depends on the
number of tissue specific and non-specific binding sites.
These effects can be lumped and
described by the partition coefficient. FGF-2's partition coefficient, hence its affinity to tissue
elements, was approximately 5 times greater than that of EGF, consistent with the binding of
FGF-2 to existing rich number of fixed heparan sulfate proteoglycan (HSPG) binding sites in
extracellular matrix. In cardiac tissue, HSPG's exist in the form of glypican [62].
These
measurements provide for the first time determination of diffusivities of FGF-2 and EGF in
myocardium. The larger diffusivity of FGF is consistent with the three-fold greater molecular
weight (17.2 kDa) of this growth factor compared to EGF (6.2 kDa). This consistency further
validated that the proposed method to determine diffusivity is sensitive enough to detect the
39
difference in diffusivities of molecules in small range of molecular weight such as that from 6-17
kDa.
Diffusivity provides a good first order estimate of the fate of the growth factor at a time
point after locally delivery to myocardial tissue if the system is diffusion dominated. In aqueous
solution, if assumed to be similar to diffusivity of myoglobin (17kDa)[63, 64], FGF-2 diffusivity
would be 94-102 um 2/sec. Diffusivity of FGF-2 in myocardium, therefore, is approximately 100
times slower than their diffusivities in water. Although transport of growth factors in the living
body may be far from that in myocardium on the diffusion cell, these diffusivity measurements
provide crucial data that will be incorporated into a computational model of myocardial transport
in the later chapter to predict spatial distribution of macromolecules in myocardial tissue in-vivo.
40
CHAPTER 4
COMPUTATIONAL MODELING OF
MACROMOLECULAR TRANSPORT IN VASCULARIZED
TISSUE
4.1 Introduction
4.1.1 Macromolecular Transport in Vascularized versus Solid Tissue
A quantitative understanding of myocardial transport is becoming of pressing importance
as we begin to look toward myocardial drug delivery for therapeutic angiogenesis[65-69]. Many
angiogenic agents have been directed against myocardial tissue to induce new vessels or enhance
collateral circulation[68]. Although different modes of local administration, e.g. intrapericardial or
intramyocardial, have been undertaken, much is unknown about the optimal delivery and actual
clinical effect of these potential angiogenic factors[65-69]. It is highly desirable to understand the
fate of the drug and the nature of its transport before attempting to deliver to the living heart. The
fate of the drug in the context of local delivery can be sufficiently described by its local spatial
distribution since its systemic pharmacokinetics and clearance is often negligible. What may not be
ignored is the neighboring tissue, i.e. the coronary arteries, since angiogenic growth factors are
potent smooth muscle mitogens that may be active when exposed to the vascular plaques at
vicinity, and could exacerbate neointima thickening[70-77].
While macromolecular transport in arteries has been studied extensively[39, 41-44], very
little is known about drug transport in myocardium. The heart develops from ontological venoarterio anastomoses and indeed bear striking structural similarities to arteries (e.g. endothelium vs.
endocardium, media vs. myocardium, vasa vasorum vs. coronary arteries), but numerous
complexities specific to the heart creates an entirely new set of concerns. For instance, cardiac
41
myocytes are arranged in a complicated three-dimensional configuration perfused by an extensive
network of capillaries. Myocardial tissue is under constant rhythmic contraction and the large
amount of blood propelled through the myocardium by convection makes it much different from
that of arteries and other solid organs. Regions of drug transport in myocardium can be roughly
divided into those that reside in the myocardial parenchyma and those that are within blood vessels
(intracapillary). Each region has unique mass transport properties. Because of the complex nature
of myocardium, each factor affecting local drug transport should be studied separately. In this
section, a computational model is used to study the role of intracapillary convection in drug
transport in myocardium, which may well be applied to other vascularized tissue. Understanding
drug transport in myocardium will provide a foundation for the rational design of safe and effective
myocardial drug delivery systems.
4.1.2 Transport Mechanisms
For drug to be effective, it must be in contact with the cells in the target tissue. The spatial
distribution of the drug, therefore, is a crucial piece of information in studying local drug delivery.
The transfer of drug from the point of the delivery device to the tissue of interest and its spatial
distribution is potentially governed by diffusion, convection and tissue binding. In this section, a
theoretical background is presented to describe these transport phenomena.
4.1.2.1 Diffusion
Diffusion is the process by which matter is transported from one part of a system to another
as a result of Brownian random molecular motions[78]. This Brownian motion has no preferred
direction. However, if one were to put molecules into a two connected chambers so that one
chamber contains a higher concentration of that molecule respect to the other and observe the
42
movement of molecules across an imaginary boundary between the two, for a given interval of
time, a fraction of molecules in the high concentration chamber move to the lower one and the
same fraction of molecules in the lower concentration chamber will move to the higher one. Thus,
as there are more molecules in the higher concentration fraction than the lower concentration one,
the net molecular flux will be from the higher concentration side to the lower one by random
molecular motion.
The quantitative treatment of the diffusion phenomenon was first described by Fourier in
1822[79] in context of heat transfer. In 1855, it was Fick who adapted the mathematical equation
of heat conduction into a quantitative description of molecular diffusion[80]. Fick's first law of
diffusion is based on the hypothesis that the rate of transfer of substances through a unit area of a
section is proportional to the concentration gradient measured normal to the section[78], i.e.
F = F=
-D 8 x
where F is the rate of transfer per unit area out of a section, C the concentration of diffusing
substance, x the space coordinate measured normal to the section, and D is diffusivity. D, for most
cases can be appropriately approximated as a constant, but in some cases could markedly depends
on concentration, for example diffusion in high polymers[78]. Fick's first law of diffusion is
analogous to Fourier's first law of heat conduction,
the thermal conductivity and
#, is the heat flux,
Oh
-2
8T
,
where T is the temperature, A is
and Newton's law of viscosity which governs the
molecular momentum transport (internal friction) which states that
43
#
, where 77 is the
,y= -i
Dy
dynamic viscosity, v, the velocity component in the x direction and , is the momentum flux in
the y direction[8 1].
While Fick's first law describes steady state molecular transport, the mathematical
treatment of the transient behavior of diffusion requires conservation laws. In general, if internal
generation or degradation of the quantity can be neglected, one can safely state that the rate at
which a quantity enters a segment is equal to the sum of the rate at which the quantity leaves and
accumulates.
If we consider a region of unit depth bounded by planes at y and y+Ay (and x and x+ Ax),
and letting C be the molecular concentration, which changes with time, and F be the molecular
flux density, the above conservation equation becomes
AxF| =AxF y+Ay
aC- AxAy.
at
Dividing by AxAy and taking the limit as Ay approaches zero, we get
.F(-F,.,
Ay->O
Ay
lim
Hence,- 8F
C
at
8C
By replacing the flux density and the property which changes with time, one can obtain
analogous differential equations for momentum and heat conservation laws. If we substitute F in
the above equation with Fick's first law of diffusion, the differential equation of diffusion can be
obtained:
44
D2C
aC
ax 2
at
4.1.2.2 Convection
Convective transport carries the molecules along with the fluid. This type of molecular
transport is represented by the term V -VCi. In fluids, V represents fluid flow velocity, and C is
the concentration of the molecules. In tissue, the convective velocity V is linearly proportional to
the local pressure gradient VP by V =
vP/p
where p is the solvent viscosity and T is the
Darcy permeability coefficient related to the tissue porosity and the effective molecular radius of
the drug[59].
4.1.2.3 Permeation
If the convective component of macromolecular transport across a membrane is negligible,
as is believed true in capillaries where the hydrostatic pressure gradient across vessel wall is
minimal, solute transport across a membrane is diffusion mediated and depends primarily on
terminal solute concentrations as:
F, = F ACj
where I, is the solute flux across the membrane, PI the solute permeability, AC the solute
concentration gradient between the two external phases. Solute permeability depends on molecular
weight, size and shape of solute, and the properties of solvent and the membrane[82].
45
4.1.3 Capillary Network in Myocardium
The myocardium is perfused by a rich network of capillaries. Transport of any substances
through myocardium depends on the molecular exchange between blood and tissue. We propose
that drug transport in myocardium differs from in solid tissue where there is no or minimal
vascularization, requiring that we analyze the effects of capillary convection on mass transport in
myocardial tissue. Definition of capillary morphology is critical for examination of myocardial
macromolecular transport, and has been studied intensively in the past century. Qualitative
descriptions of the capillary network were first attempted in the beginning of the twentieth
century[83-85]. The branching pattern of arterioles, capillaries, and venules of domestic animals
was described by Brown in 1965[86]. Microfil perfusion method was used by Bassingthwaighte to
study capillary morphometry of dog left ventricle[87]. The topology and dimensions of pig
coronary capillary network was presented in a statistical data set to provide the basis for coronary
hemodynamic analysis[88].
In general, the structural arrangements of capillary-tissue units minimize diffusion
distances between the flowing blood and the cells it serves. Muscle cells are arranged in
longitudinal arrays to facilitate its function as exerting tension on contraction. Capillaries run in
longitudinal arrays between cells, and are interconnected by shorter segments of capillaries[88,
89]. A cross section through myocardium shown in Figure 3 exemplifies a typical configuration of
the capillary network perfusing myocytes.
46
4.1 Materials and Methods
4.2.1 Transport Processes in Cardiac Tissue:
As mentioned in the introduction, regions of drug transport in myocardium can be roughly
divided into those that reside in the myocardial parenchyma and those that are within blood vessels
(intracapillary). Each region has unique mass transport properties. The flux of macromolecules
across the two regions is governed by the permeation process through endothelial cells, which
highly depends on the endothelial permeability.
Mass transport within the vascular region is governed by both convection and diffusion:
accap
_c__
at
accap
cap
ap
c
Vca pz
accap
a
( a2 c
LD
where ccap is molecular concentration within capillary, vcap
a
2
ccap +2ca
=
cp
x,
vcapy , vcp z are the capillary flows in
"+
"+
"2az
x, y, and z direction, respectively, Dcap is diffusivity of drug in blood, and t is time. Fluid velocity in
longitudinal direction along the capillary length is so much greater than in radial or circumferential
directions and the latter terms can therefore be ignored, hence the equation can be simplified to
zCcap
+cap
at
capz
z
( 2Ccap
D
=Dap
2Ccap +2Ccap
+
+
az 2
Molecular transport within the tissue region is also governed by both diffusion and
convection processes:
acL +
'+v
at
act +
act +
a+ +
'x~
'y
a2 c
acD
'=D
Z
47
'x
'+D
2
a 2c
p,2
'+D Z
a 2c,
Zaz2
v. , v, are tissue molecular convection
where ct is the molecular concentration within tissue, v,
velocity in x, y, and z direction, respectively, D, , Dx , and D, are drug diffusivity in tissue,
respectively, and t is time.
The intima fluxes between blood and tissue and vice versa are
1
j I I - is
=R
C
n d ( C ap
e~nd
Aits
cap
=R
1
C
end
cap
e~ndK
where
Jcapts
and Jts cap are molecular fluxes across endothelial layer from capillary to tissue and
vice versa, respectively, which is dependent on Rend, endothelial resistant, and
K,
partition
coefficient of drug in tissue.
Drug within the myocardium may bind to fixed tissue elements or stay free in solution
within the tissue. Since only drug in solution is freely diffusible, it is important to differentiate
between solution drug concentration, a measure of the free drug in solution, and tissue drug
concentration, a measure of the total amount of drug (free and bound) per volume of tissue. The
relationship between the two concentrations is described and determined in chapter 3 for the two
model growth factors FGF-2 and EGF for myocardial tissue:
Cb (x, y, z, t) = Cissue(xy,Z, )
K(X, y,
Z,
t)
where K is the partition coefficient which accounts for specific and non-specific binding processes.
48
4.2.2 Capillary Network Generation
The capillary network in our model was constructed from physiological dimensions and
capillary number available from anatomical measurements found in the literature[88]. Capillaries
are arranged in an idealized squared pattern in x-y plane parallel to the z-axis. The nearest parallel
vessels are inter-connected by cross-connecting capillaries randomly distributed but at locations
statistically conforming to morphometric data (Figure 12). To examine the effects of capillary
density, one can vary the mean capillary segment lengths and the number of capillaries generated.
49
/
/
4
4
r
/
40-7 1<
/
5
S
It
I
si
30-
S
a
-25-
0
S
a
20-
4.
K
a
I
4
q
4
p
4
0
4
'4
2(
41.
4
U
q
4
4
'4
10-
qa
4
a
S
S
III
20
.
5
10
1I
j/0
25
2eu
30
35
FIGURE 12: A 40x40x40 um3 vascularized tissue composed of randomly generated capillaries that
agreed statistically to measured morphormetry data embedded in the tissue block. Parallel capillaries
are arranged along z-direction connected by cross connection capillaries in both x- and y-directions.
50
4.2.3 Numerical Methods
Numerical solutions for the model are obtained by dividing the myocardium into
computational elements each with a specific tissue drug concentration. Since myocardial tissue can
generally be considered homogenous in its structural and compositional properties, partition
coefficient and diffusivity for the extravascular region was considered to be uniform. However,
their values can be significantly different in ischemic or necrotic regions.
A Crank-Nicolson[78] numerical procedure is applied on the computational grid to solve
the diffusion equation. Convective portion is discretized using the two-step MacCormack method,
which is a two-step predictor-corrector finite difference method of the Lax-Wendroff type[90]. At
each discretized point, the model can be reduced to a finite difference form dependent on the
properties of adjacent points. The model reduced to the finite difference forms as followed:
for x: capillary, x-] and x+1: tissue,
Cb(x,t+I)-Cb(x,t)
At
p Ct(x-1,t)
Ax
K
p
Ct(x+1,t)
Ax
K
for x: tissue, x-I and x+1: blood,
Ct(x,t+1)-Ct(x,t) _ P (PC (X -1,t) - Ct(Xt))
Ax
At
Ax
(Ct(X, 0 - KC
1,0)
for x-1 and x: tissue, x+1: blood,
Ct(x,t+1)-Ct(x,t) _ D
At
2Ax
(Ct(x-1,t)-Ct(xt)+Ct(x-1,t+1)+Ct(xt+1)) PCt(X,0-KC
Ax
for x and x+I: tissue, x-]: blood,
51
0)
Ct(x,t+1)-Ct(x,t)
P
IxL
LAX
(KCb
(x -1,t )
-Ct(x,t
Dt (C t(X, t) -
))
Ct(x + 1, t )+ C (x,t +1)
+ Ct(X + 1,t + 1))
2Ax
for x-] and x: blood, x+ I: tissue,
P
Cb(x,t+1)-Cb(x,t) _ D
2Axb 2 (Cb UX-
At
lt)~Cb (X,t)*
b
(X -l
+'+b(Xt
)-
Ax
(b(t
Ct (x+1,t)
K
for x and x+ 1: blood, x-]: tissue,
Cb(x, t+1)-Cb(x,t)
P (Ct(X-1, t) -Cb(xt)
Ax
K
-
At
Db2 (Cb(xt)-Cb(x+lt)+Cb(xt+1)+Cb(x+lt+))
2Ax
for x-1, x, and x+]: tissue,
C, (x,t +1)
-C,(x,t)
At
_D
2
C
1(x+,t)-2C,(x,t)+Ct(x-1,t) + C(x +1,/ +1)-
A2
2Ct(x,t +1) + Ct(x -1,t +1)
AxI
for x-], x, and x+1: blood,
C(XAt+1) - C (x,t) _ Db
At
2
2
+1)
C(x+1,t)-2Cb(xt)+Cb(x+1,Q Cb (x+1,t+1)- Cb (x,t +1)+Cb(x-1,t
AY2
where subscripts denote the spatial property of the quantity (b for blood and t for tissue), dx being
the grid size and dt the time step. These equations were shown for only x-direction. A threedimensional model was, however, generated, using the three-dimensional capillary network
configuration based on anatomical data measured experimentally, with a similar set of equations in
y- and z-directions.
52
4.2.4 Boundary Conditions and Initial Conditions
Because of the extensive computational cost, the simulation was performed on a [40 x 40 x
40] numerical grid, hence the assumption of semi-infinite length and zero-boundary condition
would not be appropriate. To analyze the effects of capillaries on local molecular transport, a zeroflux symmetric boundary condition was applied, i.e.
c(1, t)= c(O,t),
and C(n,t)=C(n +1,t) .
This imposed symmetric boundary conditions at all edges, akin to placing multiple drugrelease sources uniformly throughout the region of interest that is composed of multiple adjacent
[40 x 40 x 40] numerical cubes (Figure 13). This type of boundary condition has clinical relevance
in scenerios when local drug delivery devices, such as multiple incorporated FGF-2 heparinalginate microspheres[22], are implanted in epicardium in local vicinity of ischemic area, or
intramyocardial bolus drug injections at multiple focal sites within a small tissue area of interest.
To find the spatial drug distribution followed an implantation of local delivery devices, the
model was solved with the following initial condition:
C(x 5 ,y,,z,,t)= CS
where x ,.,Yz., are spatial coordinates of the release source, and C, is drug concentration at the
contact area of the release device and tissue, and is assumed to be constant throughout the
simulation period.
53
FIGURE 13: Zero-flux boundary condition imposes symmetric arrangement of
drug sources, equivalent to having many concatenated 40x40x40 um3 tissue
blocks surrounding the generated block (shown in white). Circular black dots
represent drug sources (not shown to scale).
54
4.2.5 Assumptions
Since capillary convection velocity is several orders of magnitude larger than diffusion
velocity in blood and even more so for that of tissue, the small step time dt required for stable
numerical solutions of the full transport equations in three-dimension would lead to costprohibitively lengthy computational times. Simplifying assumptions reduced the computational
effort. One assumption is that since capillary convection occurs so quick compared to the time
scale in which diffusion occurs that molecular concentration within intravascular region essentially
reaches the systemic drug concentration within the time step dt of diffusion. Capillaries thus
effectively behave as sinks for local molecular transport. This assumption is verified by examining
the molecular transport in a two-dimensional case of a single capillary surrounded by tissue where
the numerical solution of a full set of mass transport equations described previously is solved.
55
4.3 Results
4.3.1 Capillaries act as sinks for transport
A 25x1000 grid with dx=lum was created to examine the relation between capillary
convection and diffusion in both blood and tissue. A single 1mm-long capillary spans (x=15-20,
y=1-1000), and the surrounding grid is extravascular tissue. The molecular source is placed at
(x=12, y= 4 ). It should be noted that capillary length in this model is exaggerated to 1000um to
examine the transport phenomenon. This capillary length was chosen to be excess of the real
capillary segmental length to account for the total distance in capillary bed that the blood spend
before traversing to the venous side. This distance could be of several times that of capillary
segmental length. Due to the small time step (dt = 0.0001sec) needed for numerical stability, the
time required for the computational model to reach equilibrium is very long. Thus, only one
representative case where Dcap=100um 2/s,
Dtissue=
um2/s, vap=1000um/s, and Pintima=lum/s was
examined. These values were chosen to give the worst-case scenario within the realistic range for
capillaries to act as sinks. The resulting molecular distribution at steady state equilibrium is defined
to be the time required to achieve a 0.1% per minute change in mean concentration. Figures 14A
and 14B show the concentration profile in log scale in x and y directions, respectively. In the xdirection, the concentration profile toward the end of the capillary is almost symmetric in xdirection and about 6 times higher in the intravascular compartment than the surrounding tissue.
This implies that drug transport at points very far downstream of capillaries rely on diffusion from
the intravascular compartment to the surrounding tissue. Along the y-direction, drug concentration
in the intravascular compartment seems to reach a constant level along the length of capillary at the
distance of about 10um downstream of the source, and the plateau concentration is about 1,000
times less than that at the source. Molecular concentration on the non-source side tissue (x=21-25)
56
is 10-3 to 10-2 times less than that of the tissue side with the source. This indicated the presence of
capillary provides a barricade for molecular transport through molecular clearance by capillary
blood flow convection.
57
25
100
x
y
80
60
40
20
0
50
102
101
lapel*
10
0
p
10
2
0U
10
10-
5
10
15
20
Distance along x-direction (um)
Figure 14A: Concentration profile in x-direction is shown in the bottom panel
corresponding to the position shown on the top panel (only shown for y=1-50).
Original figure is in color.
25
25
X
y
100
80
60
40
20
50 -
0
102
10
10
0-I
0ea
bo
-
10
-
.m . . . n a
4
10
U
10
6
fl
10
100
10
10 2
10
Distance along y- direction (um)
Figure 14B: Concentration profile in y -direction is shown in the bottom panel
corresponding to the position shown on the top panel (only shown for y=1 -50,
capillary is shown in pink color). Original figure is in color.
4.3.2 Myocardial Transport Models
A three-dimensional transport model was developed to take into account properties of a
realistic coronary capillary network configuration. Capillaries in these 3D models were considered
as sinks for molecular transport, i.e. for each run (dt-O. 1 sec) their concentration was set to zero.
Since the concentration distribution turns out to be close to isometric, the results are presented in
two-dimensional spatial distribution in x-y plane at z=2 (going through the source). For all cases,
3D simulations were run to steady state equilibrium, defined as a change in mean drug
concentration of less than 0.05% per hour.
4.3.2.1 Spatial Distribution
Figure 15 shows molecular distribution at steady state equilibrium for all cases of
vascularized tissue where
Dcap
=0.1, 1, and 10 um 2 /s, and Pintima = 0.1, 1, and 10 um/s compared to
solid tissue. These values represent the range spanning one order of magnitude lower and higher
than that of physiological values for FGF-2. Transport in vascularized tissue was shown to be
much less than that of solid tissue. Although spatial concentration distribution is more dispersed as
intimal permeability decreases, the degree of its changes is unsubstantial compared to that from
vascularized tissue to solid tissue. One interesting finding is that for vascularized tissue, drug
diffusivity seems to be an immaterial factor for transport. At steady state equilibrium, drug
distribution for different values of drug diffusivities in physiological range for FGF-2 are
remarkably similar.
60
P=10
10
P=1
P=O. I
capi Ua
D= 10
D=1
D = 0.1
Figure 15: Spatial concentration distribution at steady state is shown for the plane going
through the drug source source at different values of diffusivity and capillary permeability.
Original figure is shown in color.
4.3.2.2 Total Tissue Deposition
Figure 16 shows total tissue deposition in log scale as both functions of diffusivity and
intimal permeability for vascularized tissue compared to that in solid tissue. Total tissue deposition
is defined as the summation of drug amount in the whole simulated three-dimensional grid. As
expected from the spatial concentration distribution, drug deposition is much higher for solid tissue
than vascularized tissue. Drug deposition decreases as intimal permeability increases and drug
diffusivity decreases, and the relative change as functions of permeability and diffusivity in
vascularized tissue is much less than that between solid and vascularized tissue.
62
1.OE+08
1.OE+07
1.OE+06
0
1.OE+05
0
1.OE+04
0.
0
O+0
1.00E+02
1.OE+01
D1
1.OE+00
D=1
No capillary
P=O. 1
D=0.1
P=1
P=10
I
k
Permeability
Figure 16: Total drug deposition at steady state equilibrium for different
values of diffusivity and capillary permeability. Total deposition is shown
in log-scale.
4.3.2.3 Capillary Density
Figure 17 shows spatial drug distribution as a function of capillary density. Capillary
density was varied by changing the distance between parallel capillaries and the cross-connected
capillary distance. Figure 18 shows the total tissue deposition as a function of capillary density
compared with that for solid tissue where all cases were shown for diffusivity of lum 2/s and
permeability of 0. lum/s.
64
I
EL = 86um
LL=8U2u ma
EL=294um
Figure 17: Spatial drug concentration at different values of capillary density.
Capillary density is directly proportional to total capillary length (shown as EL) per
unit tissue volume.
1000000
0
100000
0
10000
)
0
1000
100
-
10
1
I
0
0.002
0.004
0.006
0.008
0.01
0.012
0.014
Capillary density (total length/vol, um-2)
FIGURE 18: Total drug deposition as a function of capillary density
compared to solid tissue (solid black circle). Note that drug deposition is shown on log-scale
4.4 Discussion
4.4.1 Vascularized Tissue Drug Delivery
Although diffusivity may be adequate to provide a first order estimate for growth factor
distribution in myocardium in-vitro, understanding transport of macromolecules in a living heart
requires a careful analysis of growth factor pharmacokinetics. As studies of arterial media transport
suggested, physiological forces play important role in determining drug distribution in-vivo[43].
Cardiac myocytes are arranged in a complicated three-dimensional configuration perfused by a
rich capillary network. Characterizing transport through such a complex structural tissue
arrangement raises a formidable challenge but an extremely important question that requires indepth understanding before these angiogenic growth factors can be used to achieve desired clinical
goals.
Our computational results suggested that capillaries act as sinks and impede transport of
macromolecules. Intuitively, if capillaries are sinks for drugs, we would expect that one need to
deliver a greater amount of drug than if there were no capillaries to achieve a same drug
distribution. Therefore, the balance between the release, transport and clearance kinetics of growth
factors become opposing forces to govern the final drug distribution. For molecules whose
permeability through endothelial cell lining are in the range of 0.1 to 10 um/s, drug distribution at
steady state equilibrium vary minimally compared to their differences from solid tissue drug
distribution. This implies that the strategy for drug delivery to vascularized tissue regions that
contain rich capillary density, consequently, would be to have sources of drug close enough to the
desired target tissue to impose biological effects. In context of angiogenic growth factor
66
myocardial drug delivery, this means that intramyocardial delivery would be ideal if one needs to
induce sub-endocardial collaterals. Furthermore, the results imply that myocardial delivery through
pericardial surface may not achieve appropriate drug concentration adequate for biological effect in
the deeper regions of sub-endocardium.
Some interesting observations from our two-dimensional pharmacokinetic model are worth
mentioned. At steady state equilibrium, drug concentration near the capillary wall on the opposite
side
of
distance
Yd
the
source
reaches
a
relatively
constant
level
of
drug
after
a
lag
~10pm downstream of the source along the rest of the length of the capillary (Figure
14A). This imposes an important question that is whether there will be situations when the
concentration at the vicinity of the opposite wall of the source reaches a significant concentration
to be biologically active. If this turns out to be true, our assumption that capillaries behave as sinks
will be invalid, and spatial drug distribution may penetrate much further from that predicted by this
thesis and even beyond the penetration depth of that for solid tissue where diffusion is the only
mode of transport.
4.4.2 Implications
The computational results of capillary and drug permeability through endothelial-celllining dependent drug transport has several important implications:
4.4.2.1 Normal and Ischemic Myocardial Drug Transport:
Since the degree of vascularization and capillary flow in ischemic tissue regions can be
substantially less than that of normal tissue, drug penetration in ischemic tissue can be much more
than that in healthy vascularized tissue. Therefore, if the target tissue for a drug's biological effect
is in the ischemic regions, the most effective site to implant local drug delivery device is directly at
67
the target area. However, if the desired drug's target is at the vascularized tissue zones that are
border to ischemic zone, multiple local delivery devices are necessary to achieve uniform drug
distribution, and the distance between drug sources depends on the governing transport forces.
4.4.2.2 Controlled Release Device Engineering:
It may seem intuitive that one may be able to increase the penetration depth into tissue by
making a faster release kinetic controlled release device. However, our results showed that this
may not be true since even for very highly diffusive compounds, they may be cleared by capillaries
so quick as soon as being released from the drug source thus will not be able to penetrate too far
into surrounding tissue.
Therefore, as drug transport in myocardial tissue is limited by the
clearance from capillary convection, uniformity in drug concentration can only be achieve by
implanting multiple controlled release drug delivery devices. The release rate and distance between
implanted drug sources should be predicted based on drug and tissue transport properties.
4.4.2.3 Drug Engineering
Since drug properties such as diffusivity, tissue binding, and permeability play crucial roles
in drug transport, the idea of modifying its properties to improve its spatial penetration and region
of effects can be of great interest. With the advance biochemical techniques, it becomes
increasingly feasible to change a particular drug's property without affecting the drug's biological
effect. Since the permeability of drug through the intima is important determination of drug
transport in vascularized tissue, one potential approach to improve drug penetration would be to
modify the drug of interest to increase its intimal resistance and minimize its capillary clearance.
Elmalak et. al. [42] showed that one such property that can dictate the intimal drug resistance is its
68
static charge. Negatively charged Dextrans, in fact, is thirty times higher in intimal resistance than
neutral Dextrans[42].
4.4.2.4 Drug Hydrophobicity
Hydrophobic drugs were shown to partition highly in arterial media due to their high
binding affinity with tissue elements[39]. Since drugs exist in tissue in both unbound and bound
forms, only drugs that are freely to move would be available to participate in diffusion. This would
slow the transfer of drug from one point to the next, but at the same time when present in tissue
they tend to stay longer. This implies that hydrophobic drugs permeate capillary wall from blood to
tissue better by virtue of their better partitioning in tissue than blood, but at the same time their
permeability in the reverse direction from tissue to blood is less compared to hydrophilic
compounds. If this turns out to be true, drug deposition on the tissue opposite to the source side
(Figure 14A-B) may increase to a significant concentration depending on the relative differences
between the permeabilities, and our assumptions that capillaries behave as sinks should be reverified. Transport of hydrophobic drugs in myocardial tissue is determined by the intricate play
between capillary permeabilities, diffusivity, and tissue bindings, which needs to be analyzed
rigorously for effective drug delivery systems.
4.4.2.5 Temporal Evolution of Drug Deposition and Distribution
As the ultimate purpose of angiogenic local delivery therapy is to induce the growth of
blood vessels, over time the capillary density would effectively increases and its rate of growth
depends on the spatial drug distribution and myocardial tissue biological dose response curve.
Since local spatial pharmacokinetics is governed by, among other factors as suggested by the
69
computational results, capillary density and vascular permeability to drug. This implies that as the
angiogenic treatment becomes effective, the rate of drug transport might be slower. Thus, the rate
of drug penetration would also be slower. In a way, the induced biological results from the
treatment will act as a negative feedback mechanism to limit the rate of drug transfer and restrict
the spatial distribution of drug. The steady state distribution or whether there is steady state
equilibrium depends on the intrinsic property of the system of rate of drug release, tissue bindings,
diffusion, convection, permeability, drug's degradation rate and biological potency, and potential
dynamic effects of beating. One can imagine that the complete picture of myocardial drug delivery
will become complicated as more pharmacokinetic governing factors add up. The analysis of this
system should be done carefully for safe and effective myocardial local drug delivery approach.
70
CHAPTER 5
CONCLUSION
5.1 Accomplishments
This work has illustrated a novel method of determining macromolecular diffusivity for
myocardium. A demonstration was done for the two model growth factors: FGF-2 and EGF, and
their diffusivities in rat myocardium has been elucidated in-vitro using a diffusion cell. The results
have been assembled
into computational models to examine transport mechanisms of
macromolecules in myocardium in-vivo. These modeling results provided insights into formulating
pharmacologic therapies, especially angiogenic growth factor delivering to treat ischemic heart
disease.
5.2 Future Work
The work in this thesis provided some interesting insights into the nature of local
macromolecular transport in myocardium in-vivo. Capillary convection in a large range of
physiological conditions provides a drug clearance mechanism that impedes the drug penetration,
and interestingly spatial drug distributions for a large range of diffusivities converge to essentially
the same distribution. However, there are many aspects of local myocardial drug delivery that
remain unexplored. Other potential factors that could play important roles in macromolecular
transport in myocardial tissue include transmural pressure, cardiac contraction, pulsatile flow.
Understanding the isolated effects of each factor is crucial to appreciate the more complete picture
of myocardial macromolecular transport. Furthermore, hitherto the overwhelming assumption in
local drug delivery is the region of drug deposition correlates directly with the region of its
biological effect. Ideally, the assay for drug's effect should be its desired biological effect, but not
71
its location. With this knowledge, one would eventually derive a model that integrates all factors
that govern myocardial drug transport and effects, and would be able to come up with a spatial
distribution of biological effects over time for a given drug followed a given delivery modality.
72
CHAPTER 6
APPENDICES
6.1 Partition Coefficient and Diffusivity Data
6.1.1 Partition Coeffficient Data
EGF
Tissue count
Sample Weight
(mg)
Sample Volume
(mL)
Tissue count/mL
145899666.7
1050644
1710403
1807066
1253673
1078514
30.2
43.9
45.6
32.7
27.7
0.0302
0.0439
0.0456
0.0327
0.0277
14474400
357313
335208
479969
433168
264851
51.8
46.6
54.2
63.9
34.4
2843000
26179
85865
83417
93457
98411
Average Tissue
count /mL
SE Tissue
count
34789536.42
38961343.96
39628640.35
38338623.85
38935523.47
38130733.61
859887.8376
0.0518
0.0466
0.0542
0.0639
0.0344
6897934.363
7193304.721
8855516.605
6778841.941
7699156.977
7484950.921
377661.9101
73.7
52.6
45.4
48.8
54.5
0.0737
0.0526
0.0454
0.0488
0.0545
355210.3121
1632414.449
1837378.855
1915102.459
1805706.422
1509162.499
292168.4632
24411
13773
19250
70.3
34.3
54
0.0703
0.0343
0.054
347240.3983
401545.1895
356481.4815
383420.1018
13065.64772
1348
1153
27861
12768
67.9
31.8
0.0679
0.0318
410324.0059
401509.434
306
391
456
606
455
221400
2266
5212
5878
5537
2805
31.3
63.1
67.3
68.6
39.6
0.0313
0.0631
0.0673
0.0686
0.0396
72396.16613
82599.04913
87340.26746
80714.28571
70833.33333
78776.62035
3126.560133
86600
642
825
673
777
743
37.7
40.9
33.3
34.7
44.3
0.0377
0.0409
0.0333
0.0347
0.0443
17029.17772
20171.14914
20210.21021
22391.93084
16772.00903
19314.89539
1065.215531
Drug count/2uL
295048
307076
273274
28785
27724
29265
31310
27660
1233
5157
5749
5848
5990
1527
1377
1361
108
161
172
293
132
Average Drug
Count /mL
676600
FGF
Drug count/mL Average Drug
Count /mL
4462099.09
4462099.09
4462099.09
4462099.09
4462099.09
4462099.09
Tissue count
Sample Weight
(mg)
Sample Volume (mL)
53004
52327
45794
84646
62983
9.7
9.1
10.6
9
13
0.009065421
0.008504673
0.009906542
0.008411215
0.012149533
(Mean, SE)
2228664.24
2228664.24
2228664.24
2228664.24
2228664.24
(Mean, SE)
1111946.815
1111946.815
1111946.815
1111946.815
1111946.815
2228664.24
1111946.815
25494
14511
38223
37453
18176
9174
9205
5335
8184
5570
13.2
8.4
11.3
10.4
10.6
9.9
10
8
11.5
9.8
0.012336449
0.007850467
0.010560748
0.009719626
0.009906542
0.009252336
0.009345794
0.007476636
0.010747664
0.009158879
(Mean, SE)
553588.1025
553588.1025
553588.1025
553588.1025
553588.1025
553588.1025
4396
4601
4758
1779
4797
10.4
10.1
9.8
0.00971 9626
0.009439252
0.009158879
8
0.007476636
0.0071 96262
7.7
(Mean, SE)
274408.7463
274408.7463
274408.7463
274408.7463
274408.7463
274408.7463
2750
2463
2595
2585
2135
7
12
10.5
12.4
11
0.006542056
0.011214953
0.00981 3084
0.011588785
0.010280374
(Mean, SE)
134819.0681
134819.0681
134819.0681
134819.0681
134819.0681
134819.0681
1528
1052
1663
1180
1991
6.8
9
14.7
7.5
10.5
0.00635514
0.008411215
0.013738318
0.007009346
0.00981 3084
(Mean, SE)
65024.22906
65024.22906
65024.22906
65024.22906
65024.22906
65024.22906
374
757
965
808
746
7
10.7
16.3
16.4
13.3
0.006542056
0.01
0.015233645
0.015327103
0.012429907
(Mean, SE)
30126.80953
1169
12.7
30126.80953
957
10.5
1093
386
1099
15.7
13.7
14.8
30126.80953
30126.80953
30126.80953
30126.80953
0.011869159
0.009813084
0.014672897
0.012803738
0.013831776
(Mean, SE)
12678.09977
12678.09977
12678.09977
12678.09977
12678.09977
5846832.99
6152735.165
4622601,897
10063468.89
5183985.385
6373924.863
12678.09977
587
242
206
256
309
16.3
11
9.5
9.5
9.2
0.015233645
0.010280374
0.008878505
0.008878505
0.008598131
2644482.956
40174.93549
991533.3333
984935
713556.25
761467.8261
608153.0612
811929.0941
6809.903195
452280.7692
487432.6733
519495.9184
237941.25
666596.1039
472749.343
6181.624779
420357.1429
219617.5
264442.8571
223060.4839
207677.2727
267031.0513
3533.831676
240435.2941
125071.1111
121048.2993
168346.6667
202892.381
171558.7504
2044.357937
57168.57143
75700
63346.62577
52717.07317
60016.54135
61789.76234
347.9838745
98490.55118
97522.85714
74491.0828
30147.44526
79454.72973
3953.744883
9.3
15.1
10.8
15.3
11.8
0.008691589
0.01411215
0.010093458
0.014299065
0.011028037
1110.986006
38533.12883
23540
23202.10526
28833.68421
35938.04348
30009.39236
221
333
270
251
215
85843.05207
2066559.091
1848425
3619346.018
3853337.5
1834747.17
76021.33322
(Mean, SE)
3953.744883
3953.744883
3953.744883
3953.744883
3953.744883
Tissue count/mL
280.9821862
25426.88172
23596.68874
26750
17553.59477
19495.76271
22564.58559
156.5929407
6.1.2 Diffusivity Data
FGF
Time (hr)
Sqrt(t) (sec^1/2)
Partition
source count /
Coefficient
lul
source count/ um3
count
Orifice
area(um2)
Tissue
Scaled Mass (urn)
0.5
42.42640687
1.5
8894.5
8.8945E-06
53546
63585000
55.92350564
0.5
42.42640687
1.5
8894.5
8.8945E-06
64392
63585000
67.25108085
0.5
42.42640687
1.5
8894.5
8.8945E-06
36166
63585000
37.77181311
0.5
42.42640687
1.5
8894.5
8.8945E-06
11859
20820162.5
37.8256138
0.5
42.42640687
1.5
8894.5
8.8945E-06
7995
20820162.5
25.50095137
(Mean,SE)
44.85459295
2
84.85281374
1.5
8894.5
8.8945E-06
51596
63585000
53.88692333
2
84.85281374
1.5
8894.5
8.8945E-06
123274
63585000
128.7475112
2
84.85281374
1.5
8894.5
8.8945E-06
83385
63585000
87.08739248
2
84.85281374
1.5
8894.5
8.8945E-06
31207
20820162.5
99.53823509
2
84.85281374
1.5
8894.5
8.8945E-06
25708
20820162.5
81.99855634
(Mean,SE)
90.25172368
4
120
1.5
8894.5
8.8945E-06
217939
4
120
1.5
8894.5
8.8945E-06
151027
63585000
157.7327772
4
120
1.5
8894.5
8.8945E-06
25716
20820162.5
82.02407324
4
120
1.5
8894.5
8.8945E-06
110671
63585000
115.5849231
4
120
1.5
8894.5
8.8945E-06
157104
63585000
164.0796031
4
120
1.5
8894.5
8.8945E-06
101414
63585000
105.9169014
4
120
1.5
8894.5
8.8945E-06
34584
20820162.5
110.3095563
(Mean,SE)
63585000
7.4103281
12.183373
227.615749
137.6090833
27.521582
EGF
Time (hr)
Sqrt(time)
(secA1/2)
Orifice area
(um2)
Partition
Coeficient
source
count / uL
source
count/ um3
Tissue
count
0.5
42.42640687
63585000
0.26
8829.3
8.8293E-06
23935
145.3195453
0.5
42.42640687
63585000
0.26
8829.3
8.8293E-06
28917
175.5673821
0.5
42.42640687
63585000
0.26
8829.3
8.8293E-06
17436
105.8613575
0.5
42.42640687
63585000
0.26
11322.8
1.13228E-05
39132
185.2657251
0.5
42.42640687
63585000
0.26
11322.8
1.13228E-05
19084
90.3508918
0.5
42.42640687
63585000
0.26
11322.8
1.13228E-05
37444
177.2740931
0.5
42.42640687
20820162.5
0.26
11322.8
1.13228E-05
12002
173.5350555
(Mean, SE)
Scaled Mass (um)
150.4534358
2
84.85281374
20820162.5
0.26
8829.3
8.8293E-06
13502
250.3567257
2
84.85281374
63585000
0.26
8829.3
8.8293E-06
33214
201.6562932
2
84.85281374
63585000
0.26
8829.3
8.8293E-06
34565
209.8587877
(Mean, SE)
220.6239355 15.05378509
4
120
63585000
0.26
5731.2
5.7312E-06
26606
248.857592
4
120
20820162.5
0.26
5731.2
5.7312E-06
11994
342.6146373
4
120
20820162.5
0.26
5731.2
5.7312E-06
12723
363.4388886
(Mean, SE)
14.4105126
318.3037059 35.23958199
6.2 Matlab Code for Simulations of Myocardial Drug Transport
2D Simulation
clear;
colmax=25;rowmax=1000;
c(1:rowmax,1:colmax)=O;
c(10,12)=100;
Dt=0.0001;Dx=1;P=10;D=1;Dcap=100;
kP=P*Dt/D~k_D=D*Dt/DZ^2;kDcap=D-cap*Dt/Dr^2;
vrow(l:colnax)=0;vrow(16:20)=1000;
[invA1,B1=getrinvA2(vrow,colnax,k..D,kP,kDcap);
vcol(1:rowmax)=0;
[mvA2,B2] =get~nvA2(vcolrowmax,k_D,k_,kDcap);
vcol(1:rowmax)=1000;
[mvA3,B3] =get-invA2(vcolowaxkD,k,k_Dcap);
error_c=0;
for t=1:10000000000
%t
c(10,12)=100;
c=invAl*(Bl*c);
c=c';
%Diffusion in ydir
for i=1:15,
ctemp(1:rowmarx,1)=c(1:rowmax,i);
ctemp=invA2*(B2*ctemp);
c(1:rowrmaxi)=ctemp(1:rowmax,1);
end;
for i=21:colmax,
ctemp(1:rownax,1)=c(:rowmax,i);
ctemp=invA2*(B2*ctemp);
c(1:rowmax,i)=ctemp(1:rowmax,1);
end;
for i=16:20,
ctemp(:rowmax,1)=c(1:rownax,i);
ctemp=invA3*(B3*ctemp);
c(1:rowmax,i)=ctemp(1:rowmax,1);
end;
% Convection in capillary
bcl=O;bcn=O;
for i=16:20,
ctemp(1:rowmax,1)=c(1:rowmax);
bcn=c(rowmax);
[ctemp]=convldl(ctemp,rowmax,1000,bcl,bcn);
c(1:rowrmax,i)=ctemp(1:rowmax,1);
end;
if mod(t,10000)==0
t
errorc=(mean(mean(mean(c)))-mean(mean(mean(c_p))))/mean(mean(mean(cp)))*100
if error c<.0000002
break
end;
end;
%imagesc(c), drawnow;
end; %t
save([res2d_25new','c',t')
Function GetinvA2
function [nvAB]=getinvA(vnmaxkD,kP,k_Dcap);
%k_D=D*Dt/Dx'2, kP=P*Dt/Dx
%Assigning matrix components to nodes;
A(1:nmax,1:nmax)=0;B(1:nrax,l1:nmax)=0;
77
%Taking care of beginning and ending nodes:
if v(1)~=
A(1,1)=1+k Dcap;
A(1,2)=-0.5*k Dcap;
B(1,1)=1-kDcap;
B(1,2)=0.5*kDcap;
else
A(1,1)=1+k D;
A(1,2)=-0.5*kID;
B(1,1)=1-k D;
B(1,2)=0.5*kD;
end;
if v(nmax)-=0,
A(nrmax,nmax-1)=-0.5*kDcap;
A(nmax,nmax)=1+kDcap;
B(nmax,nmax-1)=0.5*kDcap;
B(nsnax,nmax)=1-k_Dcap;
else
A(nxnax,nnax-1)=-0.5*k_D;
A(nmaxnoax)=1+k_D;
B(nmax,nsax-1)=0.5*kD;
B(nmax,nsax)=1-k_D;
end;
%%%%%%%%%%%%%%%
%In-between nodes
for i=2:nmax-1,
ifv(i)~=0,
if and(v(i-1)=0,v(+1)-0),
A(i,-1)= -0.5*k Dcap;
A() = 1+kDcap;
A(ii+1)= -0.5*k Dcap;
B(i-1)= 0.5*k _Dcap;
B() = 1-kDcap;
B(ii+1)= 0.5*k _Dcap;
elseifand(v(i-1)==0,v(s+1)-0),
A(s-1)= 0;
A(,s) = 1+0.5*k Dcap;
A(s)+1)= -0.5*kDcap;
B(i,-1)= kP;
B(is) = 1 - kP - 0.5*kDcap;
B(i,+1)= 0.5*kDcap;
elseifand(v(i-1)~Ov(i+l)==0),
A(s-1)= -0.5*k Dcap;
A(is) = 1+0.5*k Dcap;
A(,i+l)= 0;
B(i-1)= 0.5*k _Dcap;*
B(s) =1 k_P -0.5 kDcap;
B(,i+1)= kP;
end;
else
ifand(v(i-1)==0,v(s+1)-=0),
A(,-1)= -0.5*k D;
A() = 1 + 0.5*kD;
A(,i+1)= 0;
B(&-1)= 0.5*k D;
5
B(&)) =1 -0. *k_D-k_P;
B(&)+1)= k_P;
elseifand(v(i-1)~0,v(s+1)==0),
A(-1)= 0;
A() = 1+0.5*kD;
*
A(i+)= -0.5 k D;
B(-1)= kP;
B(s) = 1 k- P - 0.5*kD;
B(s)+1)= 0.5*kD;
elseifand(v(i-1)== ,v(+1)==0),
A(,i-1)= -0.5*k D;
A() = 1 + kD;
A(s+)= -0.5*k
D;
*
B(-1)= 0.5 k D;
B() = 1 - k_D;
B(&+1)= 0.5*kD;
end;
end;
end;
invA=inv(A);
%save(d10r1' dir],'A','B)
78
3D Simulation
Generating Capillary Configuration and Flows
function [vlj_,vl_2,v2_1,v2_2,v3_1,v3_2,ndexk.,k2,az._nodesx_c,y_ccon,first,last,ind]=bflow3(ab,c)
%INPUT: tissue size (xy,z)
%OUTPUT: vx,vyvz (xy,z) velocity or capillary satinx
xmax=a; %rmax length in x-dir, each unit corresponds to 5umr.
ymax=b; %Amaxlength in y-dir, each unit corresponds to 5um.
zmax=c; %max length in z-dir,each unit corresponds to Sum.
%A SETTING UP TOPOLOGY FOR CAPILLARY NETWORK
%Al:
%
Output--> c..con(:,:,:)
contains cross connection topology information
% ccon(xy,z) cross connection matrix, =0 (no cross connection) =1left x-1), =2(above y+l), =3(right x+l), =4(below y-1)
c_con(1:xrrax,1.ymax,l:zmax)=0;
for x=1:4:xmax,
for y=l:4:ymax,
z_prev=0;
k_new=i;
zmax,
zodist = round(abs(randn(1)+7)); %zodist- distance between cross connection in z-dir, normally distributed range-=10-30um
while zjprev <=
zsprevrzsprev+z-dist;
if zprev <= zmax
if c.con(x,y,zoprev) == 0
c-con(x,y,zprev) = round(rand(l)*3+1);
% Making cross connections to adjacent capillaries
if c-con(xy,zprev) ==
1
if (x-4) > 0
if c-con(x-4,y,zprev) =0
ccon(x-4,y,z_prev) = 3;
else
c-con(x,y,zprev) =0;
end
else
cqcon(x,y,z-prev) =0;
end
end
if ccon(x,y,zprev) == 2
if (y+4) < ymax
if c_con(x,y+4,zprev) == 0
c_con(x,y+4,z_prev) = 4;
else
c..con(yy,zjprev) =0;
end
else
c con(x,y,z-prev) =0;
end
end
if ccon(x,y,z-prev) == 3
if (x+4) < xmax
if ccon(x+4,y,zprev) == 0
cpcon(x+4,y,z-prev) = 1;
else
c.con(x,y,z_prev) =0;
end
else
c_con(x,y,zprev) =0;
end
end
if ccon(x,y,zprev) == 4
if (y-4) >0
if c.con(x,y-4,zprev) == 0
c_con(x,y-4,zprev) = 2;
else
ccon(x,y,z-prev) =0;
end
else
ccon(x,y,zjprev) =0;
end
end
end %if
end %if
end %while
end
end
79
1=1;
for i=1:max,
for j =1ymax,
knew=1;k_prev=1;
for k=:zrmax,
if ccon(ij,k)-=0,
ifk new-=1,
dist)=k-kprev;
k-prev=k;1=1+1;
end;
end;
k-new=k-new+1;
end
end;
end
% B: GENERATING CONNECTIVITY MATRIX
%Positive flow convention: (+) in x,y and z axes.
%Bl: Calculating number of maximum nodes and z-coord of last nodes
max_nodes=0;
for ic1:4:xmax
4
for j=1: -ymax
for k=1:zmax
if ccon(i,j,k)-=0
maxnodes=maxnodes+1;
end
end
for k=zmax--1:1
if c.con(i,jk)- 0
k.max(ij)=k;
break;
end;
end;
end
end
%B2: Assigning data (length,diameter,viscosity) to nodes + calculating conductivity
% -- > length, d-->diameter, cond-->conductivity
% Units -- > CGS
It
mu= 0.05; %viscosity (in dynes.s/cm2) (1cP=10^-2 dynes.s/cm2)
n=1;
index(1:xmax,1:ymax,1:zmax)=0; %indexes of nodes given their coordinates
for i=1:4:xmax
for j=1:4-ymax
k_prev=0;
k new =1;
for k=1:zmax
ifc
con(i,j,k)~=0
if knew= =1 %tracking the first node
first(n)=1;
else first(n)=O;
end
% .a: -z dir element
lt(n).a = (k-k-prev)*5/10000;
%in cm
d(n).a=4*(1/10000)+4*(1/10000)*rand(1); %capillary diameter range 4-8um
cond(n).a=(3.14*(d(n).a)^4)/(128*mu*lt(n).a); %conductivity
index(i,j,k) =n;
% .b: +z dir element
ifk new-1
lt(n-1).b = (k-kprev)*5/10000;
d(n-1).b=d(n).a; %capillary diameter range 4-8um
cond(n-1).b=(3.14*(d(n-1).b)^4)/(128*mu*lt(n-1).b); %conductivity
end;
if k == k-max(ij)
last(n)=1;
lt(n).b = (zmax-kmax(ij))*5/10000;
d(n).b=4*(1/10000)+4*(1/10000)*rand(); %capillary diameter range 4-Bum
cond(n).b=(3.14*(d(n).a)^4)/(128*mu*lt(n).a); %conductivity
else last(n)=O0;
end;
k_prev=k;
k-new=o;
n=n+1;
end; %if
end; %for k
end; % for j
end; % for i
80
% Stores information of the compliment cross-connection nodes
n=1;
for i=1:4:xmax
4
for j=1: ynax
for k=l:zmax
if rcon(i,j,k)-=0
if ccon(,j,k)==1
ind(n)=index(i-4,j,k); %ind-->stores index of its cross-connected node
end;
if
ccon(i,j,k)==2
ind(n)=index(i,j+4,k);
end;
if c_con(ij,k)==3
ind(n)=index(i+4,j,k);
end;
if c_con(i,j,k)==4
ind(n) =index(ij -4,k);
end;
n=n+;
end;
end;
end;
end;
for i=1:maxnodes
cond().c = 0;
end
% .c: cross-connection elements
for i=l:max nodes
lt(i).c=30/10000;
d(i).c=4*(1/10000)+4*(1/10000)*rand(1); %capillary diameter range 4-8um
if (condi).c) == 0
cond(i).c=(3.14*(d(i).c)'4)/(128*mu*1t(i).c); %conductivity
cond(nd()).c=cond(i).c;
end
end;
%End B2;
%B3: Choosing random source(450-550um in z-dir) and sinks(0-100um and 900-1000um in z-dir)
n=1;nl=l;n2=1;n3=1;
for i=14:xmax
for j=1:4.ymax
for k=1:zmax
if c con(i,jk)-=0
if and((k < 0.3*zmax),first(n)-l)
sinkl(nl)=n;
nl=nl+1;
end
if and((k>0.7*zmax),ast(n)-=1)
sink2(n2)=n;
n2=n2+1;
end
if and((k>.4*zmax),(k<.5*zmax))==1
source(n3)=n;
n3=n3+1;
end
n=n+1;
end
end
end
end
i=round(rand(1)*(n1-2)+1);
skl=sink(i);
j=round(rand(1)*(n2-2)+1);
sk2=sink2(i);
k=round(rand(1)*(n3-2)+1);
sc=source(k);
%End B3;
%B4: Assigning Conductivity Matrix [G]
B(1:maxnodes)=o0;
Px=28*1333;Psk1=25*1333;P_sk2=25*1333;P_sc=40*1333;
/Px->pressure @ boundary nodes, P-sk,P-sc: pressure at sink and source
G(l:max_nodes,1:max_nodes)=0;
B(:maxnodes)=O;
for i=1:max nodes
ifand(and((i-=sk),(i-sk2)),(i-=sc))
G(,i)=cond(i).a + cond(i).b + cond().c;
ifind(i)==skl
B(i)=B()+cond(i).c*P_ski;
else
G(,ind(i))=-cond(i).c;
end;
81
ifind(i)==sk2
B(i)=B(i)+cond().c*P_sk2;
else
G(iind(i))=-cond(i).c;
end;
ifind(i)==sc
B(i)=B(r)+cond(i).c*Psc;
else
G(r,ind())=-cond(i).c;
end;
ifi+1==sc
B(i)=B(i)+cond(i).b*P_sc;
end;
ifi+1==sk2
B()=B(i)+cond(i).b*P_sk2;
end;
ifi+l==skl
B(i)=B(i)+cond(i).b*P.skl;
end;
ifi-1==skl
B()=B()+cond(i).a*Pski;
end;
if i-1==sk2
B(i)=B(i)+cond().a*P_sk2;
end;
ifi-l==sc
B(i)=B(i)+cond(i).a*Psc;
end;
iffirst(i)==i
B(i)=B(i)+cond(i).a*Pr,
else
G(ii-1)=-cond(i).a;
end;
if last()=l=
B(i)=B(i)+cond(i).b*Pr
else
G(ri+l)=-cond(i).b;
end;
end;
end
n=1;
for i=i:maxnodes
if and(and((i- =sk1),(- =sk2)),(i-=sc))
G1(n,:-)=G(i,:);
n=n+1;
end
end
n=1;clear G;
for i=1:rnax_nodes
if and(and((i-=sk1),(i~-=sk2)),(i~-=sc))
G(:.,n)=G1(:,i);
n=n+l;
end
end
n=1;
for i=1:maxnodes
if and(and((i-=skl),(-=sk2)),(i-=sc))
Bl(n)=B(i);
n=n+l;
end
end
P=(inv(G)*B1)/1333;
%Reassigning P
ni=0;
bound.n=[skl P.skl; sk2 P.sk2; sc Psc]; % nodes with pressure boundary condition
bound.n=sortrows(boundjnl);
for i=1:rnaxndes-3
ifi==boundn(i,1)
ni=nl+l;
P1(r)=bound n(1,2)/1333;
for j=i:rnax nodes-3
P1(+n1)=Pj);
end;
elseif i==bound-n(2,1)
ni=nl+1;
P1(i) =bound n(2,2)/1333;
for j =i:rnanodes-3
P1(j+n1)=POj);
end;
elseifi= =bound-n(3,1)
nl=n+1;
P1(i)=boundn(3,2)/1333;
for j =i:rnaxnodes-3
82
P1(j+nl)=POj);
end;
else
P1(i+nl)=P();
end;
end;
%clear P;
Pl=transpose(P1);
%End B4;
%B5: Calculating velocity for each segment
%velocity v -- > um/s
%vx(l:xmax,1-ymax,r:zmax)=0;
/evy(1:xmax,1-ymax,1:rmax)=0;
%vz(1:xmax,1-ymax,1:zmax)=0;
v1l1(1:max,1-ymax,1:znax)=0;
v1_.2(1:xmax,1-ymaxl:zmax)=0;
v2_1(1:xmax,1:ymaxl:max)=0;
v2_-2(1:xmax,1-ymax,1:zmax)=0;
v3_-1(1:xmax,1:yma,1:max)=0;
v3_2(1:max,1yymax,1:zmax)=0;
for i=1:maxnodes
v(i).c = 0;
end
for i=1:maxnodes
for x=1:4:xmax
for y=1:4.ymax
for z=1:zmax
ifindex(Yy,z)==i-1
if first()==1,
k_1(i)=1;
else
k_1(i)=z;
end;
end;
ifindex(x,y,z)==i+1
iflast()==1,
k_2(i)=zma;
else
k2(i)=z;
end;
end;
ifindex(x,y,z)==ind()
end;
end
end;
end;
end;
for i=1:maxnodes
for x=1:4:xmax
fory=1:4:ymax
for z=1:zmax
%
if index(x,yz)==i
%
%
%
%
xin=i;
z_n=z;
end
if index(xy,z)==i-I
%
%
k_1=z;
end
% ifindex(xy,z)==i+1
%
k_2=z;
end
%
% if index(x,y,z)==ind(i)
% x-c=r;
% y.c=y;
%
end
%v3_1: vel going into node from ( z dir
%v3_2: vel going into node from (+) z dir
%going into nodes -- > positive velocity
if index(x,y,z)==i
if first)==1
v3_1(x,y,z)=10000*cond(i).a*(Px-P1(i)*1333)/(3.14*(d(i).a/2)^2);
v3_-2(xy,z)= 10000*cond(i).b*(P1(i+1)*1333-P1(i)*1333)/(3.14*(d(i).b/2)^2);
v3_1(x,y,1:z-1)=v3_1(x,y,z);
3
v _2(x,y,1:z-1)=-v3_1(,y,r);
v3el(xyz+1:k_2(i)-1)=-v3_2(x,y,z);
v3-2(x,y,z+1:k_2(t)-l)=v3_2(x,y,z);
last(i)==1
elseif
83
v3_(xy,z) 1OOO*cond (i).a*1 (1-1) *13 33-P1 ()*1333) /(3.14*(d (1),a/ 2)2);
v3 .2(xy,z) =10000*cond (i.b*(Px-P1 (1)*1333) /(3.14*(d~i).b / 2)2);
v3 .1(xy,z+ l:zrnax)=-v3.2(y~y,z);
v3..2(xy,z+1:zmix)=v3.2(xy,z);
else
v3-(x,y,z)1OO0econd()a(PlQ-)-PlQ))*1333/(3.14*(d@l).a/2)^2);
v3....2(xy,z)lOOOO0*cond®i.b*(PlQ,+l)-Pl(1))*1333/(3.14*(d®i.b/2)^2);
v3j(x,y,z+l:k-2(-l)-v32(yz)
v3 -2(xy,z+l:k.2(i)-1)=v32(xy,z);
end;
if c....on(x,y,z)=1
vl-l(x C@s+:x-l,y,z)=V1 .(Yz)
if~~~
y..cozxy));
end
ifc c. on(xy,z) =2
v2...2(x+l~ yc()-l,z)v...2(xyz);
vl-j(xy+1.y..c)-1,z)=-vl22(x,y,z);
end
ifc~con(x,y,z)4=
v2 (xy,z)100OO*cond(i).c*(Pl(md(i))-Pl~i))*1333/(3.14*(d®i.c/2y2);
v2....(x,y....c().-,z)v21(,y,z);
v2-(x,y....c().-z)=-v21(x,y,z);
end
end;
end;
%End B5;
84
3D Transport Model
function main3d2(dlabelplabel);
nmnax=40;
if and(dlabel==1,plabel==.01),
load(rdOlpO01cap1');
elseif and(dlabel==1,plabel==.01),
load(rdlpO01cap1');
elseif and(dlabel==10,plabel==.01),
load(fd1OpOO1cap1');
elseif and(dlabel==100,plabel==.01),
load([d100pO01cap1]);
elseif and(dlabel==.1,plabel==.1),
load(fd01p0lcap 1');
elseif and(dlabel==,plabel==.1),
load(rdlpolcap']);
elseif and(dlabel==10,plabel==.1),
load(CdlOpOlcap']);
elseif and(dlabel==100,plabel==.1),
load([d1OOpO1cap1');
elseif and(dlabel==.1,plabel==1),
load([dOlp1cap1']);
elseif and(dabel==1,plabel==1),
1oad([dlp~cap1'));
elseif and(dlabel==10,plabel==1),
load(rdlOplcap1]);
elseif and(dlabel==100,plabel==1),
load([dlQOplcap1]);
end;
if and(dlabel==.1,plabe==10),
load(Cd0lpl0cap1]);
elseif and(dlabel==1,plabel==10),
load([d1p10cap 1]);
elseif and(dlabel==10,plabel==10),
load(rd10p10cap 1');
elseif and(dlabel==100,plabel==10),
load(rd100p 10cap1');
end;
load veldata_1;
c(1:nmax,1:nmax,1:nmax)=0;
c(2,2,2)=100;
cp=c;
tnax=100000;
Dt=.1;Dx=5;
r=dlabel*Dt/(Dx)^2;
for k=1:40
for count=1:40,
sinvA1600(k).x((count-1)*40+ 1:(count-1)*40+40,(count-1)*40+1:(count-1)*40+40)=sparse(MvAx(,:,(k-1)*40+count));
sinvAl600(k).y((count-l)*40+1:(count-l)*40+40,(count-l)*40+1:(count-l)*40+40)=sparse(mnvAy(:.,:,(k-l)*40+count));
sinvAl600(k).z((count-1)*40+ 1:(count-1)*40+40,(count-1)*40+1:(count-1)*40+40)=sparse(mvAz:,:,(k-1)*40+count));
sB1600(k).x((count-1)*40 +1: (count- 1) *40 +40,(count-1)*40+ 1:(count- 1)*40+40) =sparse(Bx(,:,(k- 1)*40+ count));
sB1 600(k).y((count- 1) *40+ 1: (count- 1)*40+40,(count- 1)*40+ 1: (count- 1)*40 +40) =sparse(B3y(:,:,(k-1)*40+count));
sBl600(k).z((count- 1)*40+ 1: (count- 1)*40+ 40,(count- 1)*40+ 1: (count-1)*40+40) =sparse(Bz(:,:,(k- 1)*40+count));
loop
end; % count
sc1600(k).x=sparse(zeros(1600,1));
sc1600(k).y=sparse(zeros(1600,1));
sc1600(k).z=sparse(zeros(1600,1));
sbcl600(k).x=sparse(zeros(1600,1));
sbcl600(k).y=sparse(zeros(1600,1));
sbcl600(k).z=sparse(zeros(1600,1));
end; %k loop
clear invAx invAy invAz Bx By Br %invAx1600 invAyl600 invAz1600 Bx1600 By1600 Br1600;
count_t= 1;
for i=1:nmax,
85
for j=1:nmax,
cpl(ij).X=;
cpl(i,j).y=0;
0;
cp(i,j)
cpn(i,j).x=0;
cpn(i,j).y=0;
cpn(i,j).z=0;
cl(ij).x=0;
c1(i,j).y=0;
c1(i,j).z=0;
cniD.x0;
cn(ij).y=0;
cn(i,j).z=0;
end;end;
for t1=1:tnax,%%%
cp=c;
% k dir
for k=:nmax
%Boundary conditions
c(2,2,2)=100;
%Get sc1600 from c
for count=1:40
sc1600(k).x((count-1)*40+1:count*40)=c(1:40,k,count);
end;
%Get c previous
cpl= ci;
cn;
cpn =
%Calculating new time point
sc1600(k).x=sinvA1600(k).x*(sBl600(k).x*sc600(k).x + sbcl600(k).x);
%Get c present
for count=1:40
c(1:40,k,count)=sc600(k).x((count-1)*40+1:count*40,);
end; %count
%Get boundary condition
for count=1:40
cl(k,count).x = c(1,k,count);
cn(k,count).x = c(nmaxk,count);
sbc1600(k).x((count-1)*40+1,1) = r/2*(cpl(k,count).x+c(k,count).x);
sbc1600(k).x(count*40,1)
= r/2*(cpn(k,count).x+cn(k,count).x);
end; %count
end; %k
for k=1:nmax
%Boundary conditions
c(2,2,2)=100;
%Get sc1600 from c
for count=1:40
sc1600(k).y((count-1)*40+ 1:count*40)=c(k,1:40,count);
end;
%Get c previous
cpl = c1;
cpn = cn;
%ifk==20
% sc1600(20).y(780)=100;
%end;
%Calculating new time point
sc1600(k).y=sinvAl600(k).y*(sBl600(k).y*sc600(k).y + sbcl600(k).y);
%Get c present
for count=1:40
c(k,1:40,count)=scl600(k).y((count-1)*40+1:count*40,1);
end; %count
%Get boundary condition
for count=1:40
cl(k,count).y =
c(k,1,count);
cn(k,count).y = c(k,nmacount);
sbcl600(k).y((count-l)*40+1,1) =r/2*(cpl(k,count).y+cl(k,count).y);
sbcl600(k).y(count*40,1)
= r/2*(cpn(k,count).y+cn(k,count).y);
end; %count
end; %k
for k=1:nmax
%Boundary conditions
c(2,2,2)=100;
%Get sc1600 from c
for count= 1:40
sc1600(k).z((count-1)*40+i:count*40)=c(k,count,1:40);
end;
%Get c previous
cp= ci;
86
cpn =
cn;
%ifk==20
% scl600(20).z(780)=100;
%end;
%Calculating new time point
scl600(k).z=sinvAl6O(k).z*(sB1600(k).z*scl6OO(k).z + sbc1600(k).z);
%Get c present
for count=1:40
c(k,count,1:40)=sc1600(k).z((count-l)*40+1:count*40,1);
end; %count
%Get boundary condition
for count=1:40
c1(kcount).z = c(k,count,1);
cn(k,count).z = c(k,countnmax);
sbc1600(k).z((count-1)*40+1,1) = r/2*(cpl(k,count).z+c1(k,count).z);
sbc1600(k).z(count*40,1)
= r/2*(cpn(k,count).z+cn(k,count).Z);
end; %count
end; %k
% Setting concentrations in capillaries as sink
%n =1;
for i=1:40,
for j=1:40,
for k=1:40,
if or(or(or(v1_i(i,j,k)=0 ,vl_2(i,j,k)-=0),or(v2_1(i,j,k)-=0,v2_2(i,j,k)-=0)),or(v3_1(i,j,k)-=0,v3_2(i,j,k)-=0))
c(i,j,k)=0;
else
end;
end;end;end;
%cdiag=n;
if mod(tl,100)==0
t1
error_c(countt) = (mean(mean(mean(c)))-mean(mean(mean(cp))))/mean(mean(mean(cp)))*100;
errorc(count-t)
c-t(,:,:,count~t)=c(,:,:);
if and(dlabeI==.1,plabel==.01),
save(Cresdolpool cap1,'c_t','t1','error c');
elseifand(dabel==l,plabel==.01),
save(resdlpo0 cap'],' t',t'error ');
elseifand(dlabel==10,plabel==.01),
save([resdlOpOO1_cap 1','ct','t1','error c);
elseifand(dlabel==100,plabel==.01),
save(fresdlOOpOO1_capl'],'c ,'t','errorc');
elseifand(dlabel==.1,plabel==.1),
save(Cresdolpol._cap','ct','tt',error
elseifand(dlabel==l,plabel==.1),
save(resdlp0lcap1'],'clt,'t1','error c);
elseifand(dlabel==10,plabel==.1),
save(fresdl0pOlcapl'],'c_','t','error c);
elseifand(dlabel==100,plabel==.1),
save([resd100p01_.cap1J,'c_t','t1','error c);
elseifand(dlabel==.1,plabel==1),
save([resd0lpl-cap1],'ct','t1','error c');
elseif and(dlabel==1,plabel ==1),
save(Cresd1p1_cap1'],'c_','t1','error c);
elseif and(dlabel==1,plabel ==1),
save([resdl0p1_.cap1'],'ct','tr','error c);
elseif and(dlabel== 100,plabel ==1),
save([resdlO0plcapl],'c','t','errorc');
elseif and(dlabel==.1,plabel ==10),
save(resdOlplOcap1'],'c_','ti','error c);
elseif and(dabel==1,plabel ==10),
save([resd lp1Q._cap1'],'ct','t1','error-c);
elseif and(dlabel = =1oplabel==10),
save(fresd10plO10cap1'],'_t','t','error c);
elseif and(dlabel==100,plabel==10),
save([resd100p1Ocap1'],'c_e,'t1','error-c);
end;
if errorc(count~t) < .0000001
break
end;
countt=count_t+1;
end; %mod
end; %time step
87
Getting Data Matrices for Transport Simulation
clear;
D=.1;
P=1;
Dt=.1;
Dx=5;
vel data;
v1_1(1:40,1:40,1:40)=O;v1_2(1:40,1:40,1:40)=O;
2
v 3 _1(1:40,1:40,1:40)=O;v2_2(1:40,1:40,1:40)=O;
4
v _1(1: 0,1:40,1:40)=O;v3_2(1:40,1:40,1:40)=O;
%load
xrax=40ymax=40;zmax=40;nnax=xna;
kD=D*Dt/(Dx*Dx); kP=P*Dt/D;
numn=1;
vtemp(l:xmax)=O;
% x-dir
dir='3';
count=0;
for j=1.ymax,
for k=1:zmax,
v_temp(1:xax)=0;
for i=1:xmax,
if or(or(v31(i,jk)-=O,v3_2(i,jk)- =0),or(v21(i,jk)-=0,v2_2(i,jk)-=0)),
vtemp)1;
end;
end;
count=count+1;
%call getrinvA func
[mvAB)=gerjnvA3d(v.temp,nmax,kD,kP,count);
invAx(:,:,count)=invA(:,:);
Bx(:,:,count)=B(:,:);
end;
end;
% y-dir
dir='y';
count=0;
for i=1:xmax,
for k=1:zmax,
v_temp(1:xmax)=o;
for j=:ymax,
if or(or(v3_1(ij,k) =0,v3_2(i,j,k)-=0),or(vll(,jk)-=O,v12(i,jk)-=0)),
v_tempj)=1;
end;
end;
count=count+1;
%call get invA func
[xnvA,B] =getinvA3d(vjtemp,nmaxkD,k_,count);
invAy( ,:,count) =invA(:,);
By(:,:,count) =B(:,:);
end;
end;
%
z-dir
dir=';
count=0;
for i=1:xmax,
for j=l.ymax,
v_tenp(1:xnax)=O;
for k=l:zmax,
if or(or(vl_(i,j,k)-=o0,v1_2(i,j,k)~=0),or(v2_1(i,j,k)~=0,v2_2(i,j,k)~=0)),
v_temp (k)=1;
end;
end;
count=count+1;
%call getrinvA func
[LivA,B] =getinvA3d(v-temp,nrax,kD,kP,count);
invAz(:,:,count)=invA(:,:);
Bz(:,:,count)=B(:,:);
end;
end;
save(dOlnocap_10node','invA','invAy','invAz','Bx','By','Bz);
88
Function GetinvA3d
function [A,invAB]=getjnvA3d(v,nmax,kD,k_,count);
%kD=D*Dt/Dx^2, kP=P*Dt/Dx
%Assigning matrix components to nodes;
A(1:nmax,1:nrnax)=;B(:nmax,1:nmax)=;
%Taking care of beginning and ending nodes:
ifv(1)~=O
ifv(2)==O
A(1,1)=l;
B(1,1)=1-2*k_P;
B(1,2)=k_P;
else
A(1,1)=1;
B(1,1)=1-1.5*kP;
B(1,2)=0.5*k_P
end;
else
ifv(2)==O,
A(1,1)=+k_- D;
A(1,2)=-0.5*kD;
B(1,1)=1-k_D;
B(1,2)=0.5*kD;
else
A(1,1)=1+0.5*k_D;
B(1,1)=1-0.5*k_D-k_P;
B(1,2)=kP;
end;
end;
if v(nmax)-=O,
ifsv(nmax-1)==O,
A(nmax,nmax)=1;
B(nmax,nmax-1)=kP;
B(nmax,nmax)=1-2*kP;
else
A(nmax,nmax)=1;
B(nmax,nmax-1)=1-1.5*k_P;
B(nmaxA,nmax)=k_P;
end;
else
ifv(nmax-1)==0,
A(nmax,nmax-1)=-0.5*k_D;
A(nmax,nmax)=1+kD;
B(nmax,nmax-1)=0.5*k_D;
B(nmax,nmax)=1-k_D;
else
A(nmaxnmax)=1+0.5*k_D;
B(nmax,nmax-1)=k_P;
B(nmax,nmax)=1-kP-0.5*k_D;
end;
end;
%%%%%%%%%%%%%
%In-between nodes
for i=2:nmax-1,
ifv(i)~-=
ifand(v(i-1)~Ov(i+1)-A=),
A(i,i-1)= 0;
A(i,) = 1;
A(i,+1)= 0;
B(1s-1)= 0.5*k P;
B(i,) = 1-k_P;
B(i,+1)= 0.5*k P
elseifand(v(i-1)==,v(i+1)-=0),
A(i,-1)= 0;
A(i,) = 1;
A(A+1)= 0;
B(,i-1)= k_P;
B(i,) =1 1.5*kP;
B(i,+1)= 0.5*kP;
elseifand(v(i-1)~Ov(i+1)==0),
A(,-1)= 0;
A(is) = 1;
A(i,+1)= 0;
B(,s-1)= 0.5*kP;
B(i,i) =1 1.5*k_ P
BQi+1)= kP;
else
A(i,-1)= 0;
A(,i) = 1;
A(,+1)= 0;
B(,-1)= kP;
B(i,) =1 -2kP
B(i+1)= kP;
end;
else
ifand(v(i-1)~O,v(+1)-0),
A(i,-1)= 0;
A(i,) = 1;
A(i,+1)= 0;
89
B(,-1)= kP;
B(i,) =1 -2*kP;
B(,i+1)= k P;
elseifand(v(i-1)==Cv(+1)-=0),
A(i-1)= -0.5*kD;
A(i,i) =1 + 0.5*kD;
A(,i+1)= 0;
B(,-1)= 0.5*kD;
B(,i) =1 -0.5*kD -k_P;
B(i,i+1)= kP;
elseifand(v(-1)~=0,v(i+1)==0),
A(,i-1)= 0;
A(i,i) = 1 + 0.5*kD;
A(i,+1)= -0.5*kD;
B(,i-1)= kP;
B(,i) =1 -kP -0.5*k_D;
B(,i-+1)= 0.5*kD;
else
A(,i-1)=
A(i,i) =
A(i,+1)=
B(i,i-1)=
-0.5*kD;
1 + kD;
-0.5*kD;
0.5*k_D;
B,i) =
1 - kD;
B(,i+1)= 0.5*k_D;
end;
end;
end;
invA=inv(A);
90
BIBLIOGRAPHY
1. 2002 Heart andStroke Statistical Update. 2001, American Heart Association: Dallas, Texas.
2. Schoen, F.J., Blood Vessels and The Heart, in Robbins Pathologic Basis of Disease, K.V.
Cotran RS, Collins T, Editor. 1999, W.B. Saunders Company: Philadelphia, Pennsylvania. p.
493-600.
3. Schomig, A., et al., Four-yearexperiencewith Palmaz-Schatz stentingin coronaryangioplasty
complicated by dissection with threatenedor present vessel closure. Circulation, 1994. 90(6):
p. 2716-24.
4. Ip, J.H., et al., Syndromes of acceleratedatherosclerosis:role of vascularinjury and smooth
muscle cell proliferation.J Am Coll Cardiol, 1990. 15(7): p. 1667-87.
5. Ross, R., Atherosclerosis--an inflammatory disease. N Engl J Med, 1999. 340(2): p. 115-26.
6. Ross, R., Mechanisms of atherosclerosis--areview. Adv Nephrol Necker Hosp, 1990. 19: p.
79-86.
7. Ross, R., Cell biology of atherosclerosis.Annu Rev Physiol, 1995. 57: p. 791-804.
8. van Royen, N., et al., Stimulation of arteriogenesis;a new conceptfor the treatment of arterial
occlusive disease. Cardiovasc Res, 2001. 49(3): p. 543-53.
9. Risau, W., Differentiationof endothelium. Faseb J, 1995. 9(10): p. 926-33.
10. Flamme, I. and W. Risau, Induction of vasculogenesis and hematopoiesis in vitro.
Development, 1992. 116(2): p. 435-9.
11. Gilbert, S., Developmental Biology. 5th ed. 1997, Sunderland, MA: Sinauer Associates.
12. Takahashi, T., et al., Ischemia- and cytokine-induced mobilization of bone marrow-derived
endothelialprogenitorcells for neovascularization.Nat Med, 1999. 5(4): p. 434-8.
13. Kalka, C., et al., Transplantation of ex vivo expanded endothelial progenitor cells for
therapeuticneovascularization.Proc Natl Acad Sci U S A, 2000. 97(7): p. 3422-7.
14. Tomanek, R.J., Exercise-induced coronary angiogenesis: a review. Med Sci Sports Exerc,
1994. 26(10): p. 1245-51.
15. Olivetti, G., P. Anversa, and A.V. Loud, Morphometricstudy of earlypostnataldevelopment in
the left and right ventricularmyocardium of the rat. II. Tissue composition, capillarygrowth,
and sarcoplasmicalterations.Circ Res, 1980. 46(4): p. 503-12.
16. Tomanek, R.J., et al., Compensated coronary microvascular growth in senescent rats with
thyroxine-inducedcardiachypertrophy. Am J Physiol, 1995. 268(1 Pt 2): p. H419-25.
17. Tomanek, R.J., M.K. Doty, and A. Sandra, Early coronary angiogenesis in response to
thyroxine: growth characteristicsand upregulation of basicfibroblastgrowthfactor.Circ Res,
1998. 82(5): p. 587-93.
18. Baroldi, G., et al., Morphology of acute myocardial infarction in relation to coronary
thrombosis. Am Heart J, 1974. 87(1): p. 65-75.
19. Gorge, G., et al., Microvascular and collateral adaptation in swine hearts following
progressive coronaryartery stenosis. Basic Res Cardiol, 1989. 84(5): p. 524-35.
20. Klagsbrun, M. and Y. Shing, Heparinaffinity of anionic and cationic capillaryendothelialcell
growth factors: analysis qf hypothalamus-derived growth factors and fibroblast growth
factors. Proc Natl Acad Sci U S A, 1985. 82(3): p. 805-9.
21. Yu, C., A. English, and E.R. Edelman, Growth Factor Delivery Strategies, in Angiogenesis
and CardiovascularDisease, M. Simons, Editor. 1999, Oxford University Press: New York. p.
238-257.
91
22. Lopez, J.J., et al., Basicfibroblast growth factor in a porcine model of chronic myocardial
ischemia: a comparison of angiographic,echocardiographicand coronaryflow parameters.J
Pharmacol Exp Ther, 1997. 282(1): p. 385-90.
23. Raj anayagam, M. A., et al., Intracoronarybasic fibroblast growth factor enhances myocardial
collateralperfusion in dogs. J Am Coll Cardiol, 2000. 35(2): p. 519-26.
24. Shou, M., et al., Effect of basicfibroblastgrowth factor on myocardialangiogenesisin dogs
with mature collateralvessels. J Am Coll Cardiol, 1997. 29(5): p. 1102-6.
25. Laham, R.J., et al., Therapeutic Angiogenesis Using Basic Fibroblast Growth Factor and
Vascular Endothelial Growth Factor Using Various Delivery Strategies. Curr Interv Cardiol
Rep, 1999. 1(3): p. 228-233.
26. Sato, K., et al., Efficacy of intracoronaryversus intravenousFGF-2 in a pig model of chronic
myocardial ischemia. Ann Thorac Surg, 2000. 70(6): p. 2113-8.
27. Laham, R.J., D. Hung, and M. Simons, Therapeutic myocardial angiogenesis using
percutaneousintrapericardialdrug delivery. Clin Cardiol, 1999. 22(1 Suppl 1): p. 16-9.
28. Landau, C., A.K. Jacobs, and C.C. Haudenschild, Intrapericardialbasic fibroblast growth
factor induces myocardial angiogenesis in a rabbit model of chronic ischemia. Am Heart J,
1995. 129(5): p. 924-3 1.
29. Lazarous, D.F., et al., Pharmacodynamics of basic fibroblast growth factor: route of
administrationdetermines myocardialandsystemic distribution.Cardiovasc Res, 1997. 36(1)
p. 78-85.
30. Unger, E.F., et al., Basicfibroblast growth factor enhances myocardial collateralflow in a
canine model. Am J Physiol, 1994. 266(4 Pt 2): p. H1588-95.
3 1. Watanabe, E., et al., Effect of basicfibroblastgrowthfactor on angiogenesis in the infarcted
porcine heart. Basic Res Cardiol, 1998. 93(1): p. 30-7.
32. Lincoff, A.M., et al., Sustained local delivery of dexamethasone by a novel intravascular
eluting stent to prevent restenosis in the porcine coronary injury model. J Am Coll Cardiol,
1997. 29(4): p. 808-16.
3 3. Lambert, T.L., et al., Localized arterialwall drug delivery from a polymer-coated removable
metallic stent. Kinetics, distribution,and bioactivity offorskolin. Circulation, 1994. 90(2): p.
1003-11.
34. van der Giessen, W.J., et al., Heparin-coatingof coronary stents. Semin Interv Cardiol, 1998.
3(3-4): p. 173-6.
35. Slepian, M.J., Polymeric endoluminal paving. A family of evolving methods for extending
endoluminal therapeuticsbeyond stenting. Cardiol Clin, 1994. 12(4): p. 715-37.
36. Okada, T., D.H. Bark, and M.R. Mayberg, Localized release ofperivascularheparin inhibits
intimalproliferationafter endothelialinjury without systemic anticoagulation.Neurosurgery,
1989. 25(6): p. 892-8.
37. Dinbergs, I.D., L. Brown, and E.R. Edelman, Cellularresponse to transforminggrowthfactorbeta] and basicfibroblastgrowthfactor depends on release kinetics and extracellularmatrix
interactions.J Biol Chem, 1996. 271(47): p. 29822-9.
3 8. Harada, K., et al., Vascular endothelialgrowth factor administrationin chronic myocardial
ischemia. Am J Physiol, 1996. 270(5 Pt 2): p. H1791-802.
39. Creel, C.J., M.A. Lovich, and E.R. Edelman, Arterialpaclitaxel distributionand deposition.
Circ Res, 2000. 86(8): p. 879-84.
40. Lovich, M.A., L. Brown, and E.R. Edelman, Drug clearance and arterialuptake after local
perivasculardelivery to the rat carotidartery. J Am Coll Cardiol, 1997. 29(7): p. 1645-50.
92
41. Lovich, M.A. and E.R. Edelman, Mechanisms of transmural heparin transport in the rat
abdominalaorta after local vasculardelivery. Circ Res, 1995. 77(6): p. 1143-50.
42. Elmalak, 0., M.A. Lovich, and E. Edelman, Correlationof transarterialtransportof various
dextrans with their physicochemicalproperties. Biomaterials, 2000. 21(22) p. 2263-72.
43. Hwang, C.W., D. Wu, and E.R. Edelman, Physiological transport forces govern drug
distributionfor stent-baseddelivery. Circulation, 2001. 104(5): p. 600-5.
44. Lovich, M.A. and E.R. Edelman, Computational simulations of local vascular heparin
deposition and distribution.Am J Physiol, 1996. 271(5 Pt 2): p. H2014-24.
45. Hwang, C.W. and E.R. Edelman, Arterial ultrastructure influences transport of locally
delivered drugs. Circ Res, 2002. 90(7): p. 826-32.
46. Hagiwara, A., et al., Endoscopic local injection of a new drug-deliveryformat ofpeplomycin
for superficialesophageal cancer: a pilot study. Gastroenterology, 1993. 104(4): p. 103 7-43.
47. Stout, S.A. and H. Derendorf, Local treatment of respiratoryinfections with antibiotics.Drug
Intell Clin Pharm, 1987. 21(4): p. 322-9.
48. Mahoney, M.J. and W.M. Saltzman, Controlledrelease ofproteins to tissue transplantsfor the
treatment of neurodegenerativedisorders.J Pharm Sci, 1996. 85(12): p. 1276-8 1.
49. See, W.A. and Q. Xia, Regional chemotherapy for bladder neoplasms using continuous
intravesical infusion qf doxorubicin: impact of concomitant administration of dimethyl
sulfoxide on drug absorptionandantitumor activity. J Natl Cancer Inst, 1992. 84(7): p. 510-5.
50. Kuo, P.Y., J.K. Sherwood, and W.M. Saltzman, Topical antibody delivery systems produce
sustained levels in mucosal tissue and blood. Nat Biotechnol, 1998. 16(2): p. 163-7.
5 1. Hsing, J., QuantificationqfMyocardialMacromolecularTransport, in ElectricalEngineering
and Computer Science. 2000, MIT: Cambridge. p. 68.
52. Page, E.a.B., R. S., CatHeartMuscle in Vitro: Diffusion through a sheet of right ventricle. The
Journal of General Physiology, 1964. 47: p. 1129-1140.
53. Safford, R.E. and J.B. Bassingthwaighte, Calcium diffusion in transientand steady states in
muscle. Biophys J, 1977. 20(1): p. 113-36.
54. Suenson, M., DR. Richmond, and J.B. Bassingthwaighte, Diffusion of sucrose, sodium, and
water in ventricular myocardium. Am J Physiol, 1974. 227(5): p. 1116-23.
55. Safford, R.E., E.A. Bassingthwaighte, and J.B. Bassingthwaighte, Diffusion of water in cat
ventricularmyocardium. J Gen Physiol, 1978. 72(4): p. 513-38.
56. Wan, W.K., et al., Measurement of drug distribution in vascular tissue using quantitative
fluorescence microscopy. J Pharm Sci, 1999. 88(8): p. 822-9.
57. Markwell, M.A., A new solid-state reagent to iodinateproteins. I. Conditionsfor the efficient
labeling of antiserum. Anal Biochem, 1982. 125(2): p. 427-32.
58. Bolton, A.E. and W.M. Hunter, The labelling qf proteins to high specific radioactivitiesby
conjugation to a 1251-containingacylatingagent. Biochem J, 1973. 133(3): p. 529-39.
59. Deen, W.M., Analysis qf Transport Phenomena. 1998, New York: Oxford University Press,
Inc. 597.
60. Syrovy, I. and Z. Hodny, Staining and quantificationofproteins separatedby polyacrylamide
gel electrophoresis.J Chromatogr, 1991. 569(1-2): p. 175-96.
61. Dunbar, B.S., H. Kimura, and T.M. Timmons, Protein analysis using high-resolution twodimensionalpolyacrylamidegel electrophoresis.Methods Enzymol, 1990. 182: p. 441-59.
62. Asundi, V.K., et al., Developmental and cell-type-specific expression of cell surface heparan
sulfate proteoglycansin the ratheart. Exp Cell Res, 1997. 230(1): p. 145-53.
93
63. Muramatsu, N. and A.P. Minton, Tracer diffusion ofglobularproteins in concentratedprotein
solutions. Proc Natl Acad Sci U S A, 1988. 85(9): p. 2984-8.
64. Riveros-Moreno, V. and J.B. Wittenberg, The self-diffusion coefficients of myoglobin and
hemoglobin in concentratedsolutions. J Biol Chem, 1972. 247(3): p. 895-901.
65. Kellar, R.S., et al., Scaffold-based three-dimensional human fibroblast culture provides a
structural matrix that supports angiogenesis in infarcted heart tissue. Circulation, 2001.
104(17): p. 2063-8.
66. Yau, T.M., et al., Enhancedmyocardialangiogenesisby gene transfer with transplantedcells.
Circulation, 2001. 104(12 Suppl 1): p. 1218-22.
67. Bao, J., et al., Intramyocardial delivery of FGF2 in combination with radio frequency
transmyocardialrevascularization.Catheter Cardiovasc Interv, 2001. 53(3): p. 429-34.
68. Tabibiazar, R. and S.G. Rockson, Angiogenesis and the ischaemic heart. Eur Heart J, 2001.
22(11): p. 903-18.
69. Tomita, N., et al., Novel molecular therapeutic approach to cardiovasculardisease based on
hepatocyte growthfactor. J Atheroscler Thromb, 2000. 7(1): p. 1-7.
70. Reynolds, L.P. and D.A. Redmer, Expression of the angiogenicfactors,basicfibroblastgrowth
factor and vascular endothelialgrowthfactor, in the ovary. J Anim Sci, 1998. 76(6): p. 167 181.
71. Su, E.J., et al., Mitogenic effect of angiotensin II on rat carotid arteriesand type II or III
mesenteric microvessels but not type I mesenteric microvessels is mediated by endogenous
basicfibroblastgrowthfactor. Circ Res, 1998. 82(3): p. 321-7.
72. Neufeld, G., et al., Vascular endothelialgrowth factor and its receptors. Prog Growth Factor
Res, 1994. 5(1): p. 89-97.
73. DiCorleto, P.E., Cellularmechanisms of atherogenesis.Am J Hypertens, 1993. 6(11 Pt 2): p.
314S-318S.
74. Reidy, M.A., J. Fingerle, and V. Lindner, Factors controlling the development of arterial
lesions after injury. Circulation, 1992. 86(6 Suppl): p. 11143-6.
75. Nilsson, J., Smooth muscle cells in the atheroscleroticprocess. Acta Med Scand Suppl, 1987.
715: p. 25-3 1.
76. Schwartz, S.M. and C.M. Gajdusek, Growthfactorsand the vessel wall. Prog Hemost Thromb,
1982. 6: p. 85-112.
77. DiCorleto, P.E. and G.M. Chisolm, 3rd, Participationof the endothelium in the development of
the atheroscleroticplaque. Prog Lipid Res, 1986. 25(1-4): p. 365-74.
78. Crank, J., The Mathematics of Diffusion. 1979, Oxford, England: Clarendon Press.
79. Fourier, J.B., Theorie analytique de la chaleur. 1822, New York 1955: Dover Publ.
80. Fick, A., Annln Phys, 1855. 170: p. 59.
81. Bird, R.B., TransportPhenomena. 2002, New York: J. Wiley.
82. Craig, L.C., DifferentialDialysis. Science, 1964. 144(3622): p. 1093-1099.
83. Nussbaum, A., Uber das Gefass-system des Herzens. Arch. Mikrobiol. Anat., 1912. 80: p. 450477.
84. Spalteholz, W., Die Koronararteriendes Herzens. Verh. Anat. Ges., 1907. 21: p. 141-153.
85. Wearn, J.T., The extent of the capillary bed of the heart. J. Exp. Med., 1928. 47: p. 273-292.
86. Brown, R., The pattern of the microcirculatorybed in the ventricularmyocardium of domestic
mammals. Am. J. Anat., 1965. 1163: p. 355-374.
87. Bassingthwaighte, J.B., T. Yipintsoi, and R.B. Harvey, Microvasculature of the dog left
ventricularmyocardium. Microvasc Res, 1974. 7(2): p. 229-49.
94
8 8. Kassab, G. S. and Y. C. Fung, Topology and dimensions ofpig coronary capillarynetwork. Am
J Physiol, 1994. 267(1 Pt 2): p. H319-25.
89. Batra, S. and K. Rakusan, Morphometric analysis of capillary nets in rat myocardium. Adv
Exp Med Biol, 1990. 277: p. 377-85.
90. Hoffman, J.D., NumericalMethodsfor Engineersand Scientists. 1992: McGraw-Hill, Inc.
95