1 Quiz 3 solutions are in order of the questions for... (1). Correct answer B The wavelength obeys the relation
advertisement
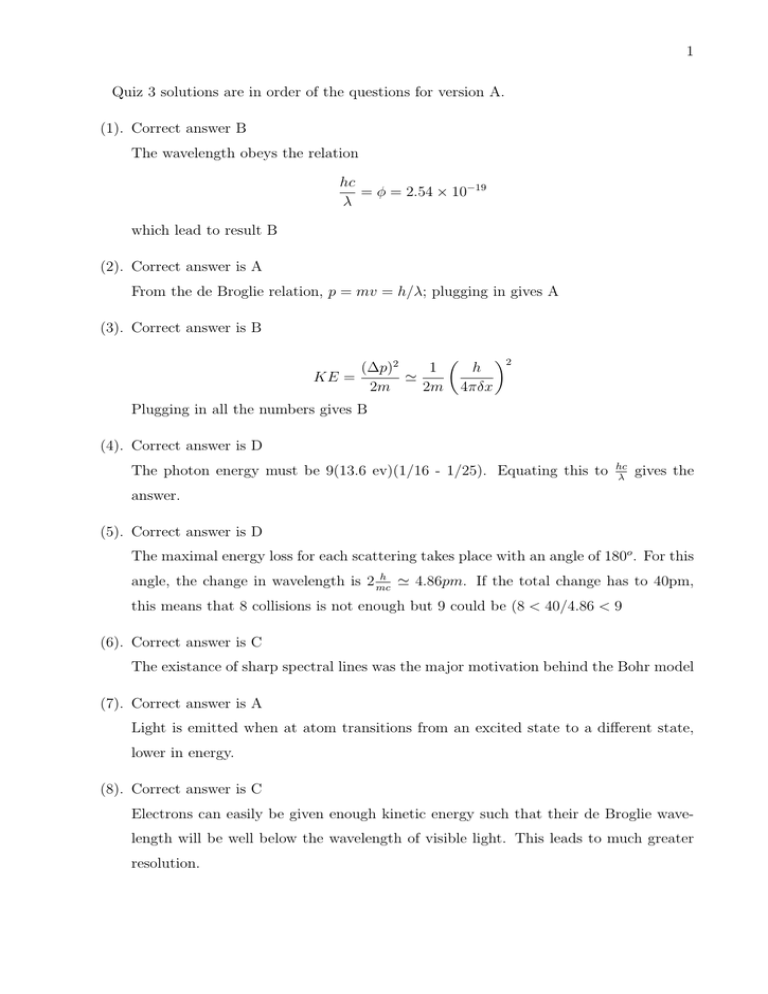
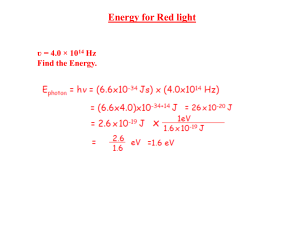
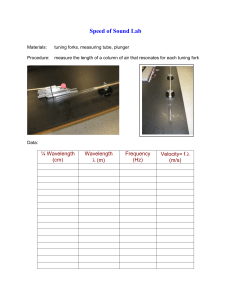
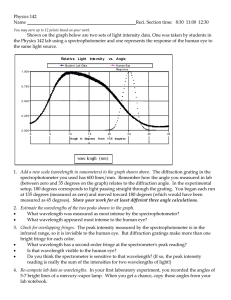
1 Quiz 3 solutions are in order of the questions for version A. (1). Correct answer B The wavelength obeys the relation hc = φ = 2.54 × 10−19 λ which lead to result B (2). Correct answer is A From the de Broglie relation, p = mv = h/λ; plugging in gives A (3). Correct answer is B (∆p)2 1 KE = ' 2m 2m h 4πδx 2 Plugging in all the numbers gives B (4). Correct answer is D The photon energy must be 9(13.6 ev)(1/16 - 1/25). Equating this to hc λ gives the answer. (5). Correct answer is D The maximal energy loss for each scattering takes place with an angle of 180o . For this h angle, the change in wavelength is 2 mc ' 4.86pm. If the total change has to 40pm, this means that 8 collisions is not enough but 9 could be (8 < 40/4.86 < 9 (6). Correct answer is C The existance of sharp spectral lines was the major motivation behind the Bohr model (7). Correct answer is A Light is emitted when at atom transitions from an excited state to a different state, lower in energy. (8). Correct answer is C Electrons can easily be given enough kinetic energy such that their de Broglie wavelength will be well below the wavelength of visible light. This leads to much greater resolution. 2 (9). Correct answer is A This atom has a completely filled n=2 level and hence is probably inert. (10). Correct answer is B By energy conservation, KE = 6.4 - 13.6/4 =3 ev