Physical Chemistry Lecture 11 Reversibility and Work, Enthalpy
advertisement

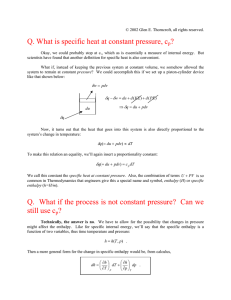
Physical Chemistry Lecture 11 Reversibility and Work, Enthalpy Mechanical work Work on the system w = − ∫P ext dV path Depends on how the external pressure is varied in the process, i.e. it depends on the path Three experimentally known possibilities P > Pext along the path: volume expands; w < 0 P = Pext, no change in the volume; w = 0 P < Pext, volume decreases; w > 0 Only ones seen Volume never increases when Pext > P Work and reversibility Consider a process that takes an ideal gas from one state to another in several different ways As the number of steps goes up, the work approaches a constant value In the limit of infinite number of steps, P=Pext at every point in the process Reversibility System remains at equilibrium with the surroundings at every point in a process A special, unique path The system does maximum work in going from the initial to the final state along a reversible path Can never be achieved with real processes Convenient for calculation of state-function changes, since these are independent of path Heat transfer at constant pressure Chemists frequently carry out processes at constant pressure, rather than at constant volume ∆UP ≠ qP Heat transfer is easy to measure What is heat transfer at constant P? Enthalpy – a new state function H, enthalpy, = U + PV A state function because U, P and V are Differential form dH = dU + PdV + VdP = dqrev − PdV + PdV = dqrev + VdP + VdP At constant pressure dH = dqrev At constant pressure, the reversible heat transferred is the change in enthalpy Differential of the enthalpy In any process, one may evaluate the change in a state function by integration state 2 ∆H = H (T2 , P2 ) − H (T1 , P1 ) = ∫ dH state1 Define the differential in terms of other changes dH ∂H = dT ∂T P ∂H + dP ∂P T Must know the functional form of the partial derivatives to do the integral Derivatives of the enthalpy Enthalpy change at constant pressure = qP, therefore ∂H ∂T P = CP The other derivative is determined from the equation of state ∂H ∂P T = V ∂V − T ∂T P Molar heat capacities, CPm, of organic liquids at 298.15 K Compound CPm (J K-1 mol-1) Compound CPm (J K-1 mol-1) n-Hexane 195.0 Benzene 135.6 n-Heptane 224.7 Toluene 156.1 n-Octane 254.0 Ethylbenzene 186.2 n-Nonane 284.5 p-Xylene 182.4 n-Decane 314.7 1,2,3-Trimethylbenzene 216.7 n-Undecane 345.2 1,2,4-Trimethylbenzene 215.9 n-Dodecane 375.3 1,3,5-Trimethylbenzene 209.6 n-Tridecane 406.7 n-Tetradecane 438.5 n-Pentadecane 469.4 n-Hexadecane 501.7 Data from the Handbook of Chemistry and Physics, 63rd Edition, CRC Press, Boca Raton, Florida Relationship between the heat capacities Example of the use of calculus Use definition of the enthalpy H = U + PV Derivative with respect to temperature ∂V ∂U P + = ∂T P ∂T P ∂V ∂P ∂V = CV + T − P ∂T P ∂T V ∂T P ∂P ∂V = CV + T ∂T V ∂T P ∂H ∂T P CP ∂V + P ∂T P Calculation of enthalpy changes in processes Constant-pressure process ∆H ∂H = ∫ dT ∂T P T1 T2 = T2 ∫C P (T ) dT T1 Isothermal process ∆H = ∂H ∫P ∂P T dP = 1 P2 ∂V ∫P V − T ∂T P dP 1 P2 Summary Calculation of state-function changes in reversible processes, even if the actual process is irreversible State functions depend on two variables Integrate the two components separately and sum to obtain total change All changes calculated with a similar formalism