Water Properties (from a Biological perspective)
advertisement

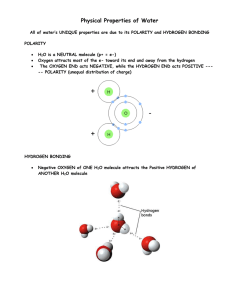
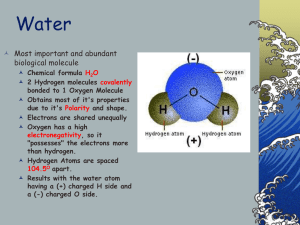
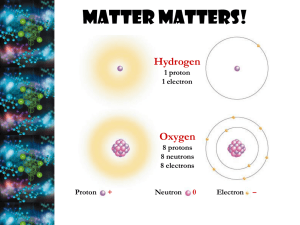
Water Properties (from a Biological perspective) Importance of Water Fills and surrounds cells 2/3 of human body Required for chemical rxns Maintains homeostasis Essential for life! A Water Molecule 1 oxygen atom and 2 hydrogen atoms joined by covalent bonds Polarity of Water Polar - electrons are not shared equally Oxygen end of water molecule attracts electrons more, giving it a negative charge H2O molecules “stick” together forming weak H bonds Cohesion of Water Water is “sticky” When water is attracted to other water molecules, cohesion occurs allows H2O to form drops, thin films, and have surface tension Adhesion of Water When water is attracted to non-water molecules, adhesion occurs Adhesion enables water to travel from roots through stems to leaves of a plant Water has a High Specific Heat Water resists changes in temperature H2O is critical to homeostasis – Ex. Near constant body temperature maintained Solubility of Water Many substances are soluble (easily dissolve) in water Water is called a universal solvent Essential for homeostasis – Ex. Transport glucose into cells – Ex. Move substances within the cell – Ex. Carry wastes out of cell H2O is essential for chemical reactions / metabolism and the chemistry of life!