Interaction of nickel and manganese in accumulation
advertisement

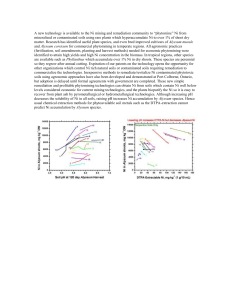
Plant Soil (2009) 314:35–48 DOI 10.1007/s11104-008-9703-4 REGULAR ARTICLE Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum murale and Alyssum corsicum C. Leigh Broadhurst & Ryan V. Tappero & Timothy K. Maugel & Eric F. Erbe & Donald L. Sparks & Rufus L. Chaney Received: 15 January 2008 / Accepted: 24 June 2008 / Published online: 2 August 2008 # US Government 2008 Abstract The genus Alyssum contains >50 Ni hyperaccumulator species; many can achieve >2.5% Ni in dry leaf. In soils with normal Mn levels, Alyssum trichome bases were previously observed to accumulate Ni and Mn to high levels. Here we report concentration and localization patterns in A. murale and A. corsicum grown in soils with nonphytotoxic factorial additions of Ni and Mn salts. Four leaf type subsets based on size and age accumulated Ni and Mn similarly. The greatest Responsible Editor: Fangjie J. Zhao. Use of trade or proprietary names or trademarks herein is for informational purposes only and does not imply any advertisement or endorsement by the US Department of Agriculture, University of Maryland, or University of Delaware. Electronic supplementary material The online version of this article (doi:10.1007/s11104-008-9703-4) contains supplementary material, which is available to authorized users. C. Leigh Broadhurst (*) : R. L. Chaney US Department of Agriculture Henry A. Wallace Agricultural Research Center, Environmental Management and Byproduct Utilization Laboratory, Animal and Natural Resources Institute, Bldg. 007, Beltsville, MD 20705, USA e-mail: leigh.broadhurst@ars.usda.gov R. V. Tappero : D. L. Sparks Plant and Soil Sciences Department, University of Delaware, Newark, DE, USA Mn accumulation (10 times control) was observed in A. corsicum with 40 mmol Mn kg−1 and 40 mmol Ni kg−1 added to potting soil. Whole leaf Ni concentrations decreased as Mn increased. Synchrotron X-ray fluorescence mapping of whole fresh leaves showed localized in distinct high-concentration Mn spots associated with trichomes, Ni and Mn distributions were strongly spatially correlated. Standard X-ray fluorescence point analysis/mapping of cryofractured and freeze-dried samples found that Ni and Mn were co-located and strongly concentrated only in trichome bases and in cells adjacent to trichomes. Nickel concentration was also strongly spatially correlated with sulfur. Results indicate that maximum Ni phytoextraction by Alyssum may be reduced in soils with higher phytoavailable Mn, and suggest that Ni hyperaccumulation in Alyssum species may have developed from a Mn handling system. T. K. Maugel Laboratory for Biological Ultrastructure, Department of Biology, University of Maryland, College Park, USA E. F. Erbe Electron Microscopy Unit, Soybean Genomics and Improvement Laboratory, Plant Sciences Institute, USDA Beltsville, Beltsville, MD, USA 36 Keywords Alyssum . Hyperaccumulator . Manganese localization . Nickel localization . Phytoremediation . trichomes Introduction More than 400 plant species are known to naturally accumulate high levels of metals such as Cd, Cu, Co, Mn, Ni and Zn. Hyperaccumulation is defined as accumulation of >1000 μg g−1 in plant dry material for Ni, and Co, and >10,000 μg g−1 for essential trace elements such as Mn and Zn which are required in larger amounts to support normal metabolism (Baker et al. 2000). The genus Alyssum (Brassicaceae) contains more than 50 Ni hyperaccumulator species, many of which can achieve 3% dry weight Ni in leaf biomass (Reeves 1992). Our research consortium has developed commercially feasible phytoremediation and phytomining technologies that can potentially clean up Nicontaminated soils (Chaney et al. 1999, 2007; Li et al. 2003a, b). The technology employs the Ni-hyperaccumulating species Alyssum murale and A. corsicum to phytoextract Ni from a range of soil types. These species are endemic to serpentine (ultramaficderived) soils throughout Mediterranean Europe, but unlike many serpentine-endemic species, they grow rapidly and prolifically, even in nonnative environments (Li et al. 2003a, b; Broadhurst et al. 2004a; Bani et al. 2007). Further, A. murale and A. corsicum can hyperaccumulate Ni in nonserpentine soil types, such as limestone and organic soils (Küpper et al. 2001; Li et al. 2003a, b; Broadhurst et al. 2004a). Several other hyperaccumulator species such as Berkheya coddi (Robinson et al. 2003) and A. serpyllifolium (De La Fuente et al. 2007) have potential for phytoremediation/phytomining but have not yet been adequately field tested. In a field test where climatic conditions permitted growth of both Alyssum and Berkheya, Berkheya accumulated <50% of the Ni concentration that Alyssum achieved (Chaney et al. 2007). Ni localization patterns have been determined for 12 Alyssum Ni hyperaccumulator species/ecotypes (Krämer et al. 1997; Küpper et al. 2001; Psaras et al. 2000; Kerkeb and Krämer 2003; Marmiroli et al. 2003; Broadhurst et al. 2004a, b; McNear et al. 2005; Asemaneh et al. 2006; De La Fuente et al. 2007; Smart et al. 2007; Tappero et al. 2007). Nickel is mainly stored in the leaves, and is particularly concentrated in Plant Soil (2009) 314:35–48 epidermal cell vacuoles, trichome bases, and the lower parts of the trichome pedicle. The upper and lower leaf surfaces of Alyssum sp. are covered with an overlapping network of branching, stalked bifurcate or stellate trichomes (Inamdar 1983; Oran 1996; Broadhurst et al. 2004b). Trichomes are anchored in the epidermal layer with a ~20 μm smooth cylindrical pedicle which has a broad fan-shaped basal compartment. In A. murale, the trichome is unicellular. The trichome basal compartment and the epidermal cells adjacent to the trichome basal compartment strongly concentrate Ni, but only one (Smart et al. 2007) of twelve studies has reported appreciable Ni in the trichome rays or nodules. In soils with normal Mn levels, Ni and Mn can be simultaneously accumulated to very high levels within Alyssum trichome bases (Broadhurst et al. 2004a, b; McNear et al. 2005; Tappero et al. 2007) although the average leaf Mn concentration is below the accepted threshold defining hyperaccumulation. On the Alyssum leaf surface, Tappero et al. (2007) found Mn concentrated in a ring-like pattern surrounding the trichome base. To further quantify potential interactions between Ni and Mn, we have investigated A. murale and A. corsicum grown in soils with factorial additions of Ni and Mn salts. Materials and methods Horticulture Alyssum murale ‘Kotodesh’ (from Albania) and Alyssum corsicum (from Turkey) were started from seed 14 Oct. 2005 in a greenhouse at USDA Beltsville. Forty-four days later seedlings were transplanted to prepared and equilibrated 500 ml pots containing Promix® soil with an increasing series of NiSO4·6H2O (0, 10, 40 mmol Ni kg−1) and/or MnSO4·H2O additions (0, 10, 40 mmol Mn kg−1) such that every combination was represented (c.f. Table 1). Carbonates (half CaCO3 and half MgCO3) were added to each pot at amounts equimolar with NiSO4 + MnSO4 to buffer the acidity generated by the metal salt additions. Initial soil pH was uniformly 6.4. Duplicate controls were prepared to better establish background Mn accumulation in Alyssum. The experiments were conducted in a greenhouse under controlled temperature and light conditions and ambient humidity. Photoperiod was 15:9 h day/night. Plant Soil (2009) 314:35–48 37 Table 1 Nickel and manganese concentrations in Alyssum murale ‘Kotodesh’ and Alyssum corsicum leaves, mg kg−1 dry weight from a composite of apical and distal leaves sampled in early spring Treatment Ni0/Mn0a Ni0/Mn0a Ni0/Mn10 Ni0/Mn40 Ni10/Mn0 Ni10/Mn10 Ni10/Mn40 Ni40/Mn0 Ni40/Mn10 Ni40/Mn40 A. murale A. corsicum Ni Mn Ni Mn 30 45 92 32 10,100b 6,347b 5,930b 15,100b 12,300b 8,638 149 159 597 383 277 382 322 269 439 767 21 16 36 61 8,770b 9,330b 5,380b 15,300b 13,900b 12,600 96 80 334 306 187 285 602 257 695 924 Treatment Ni/Mn designates mmolkg−1 Ni and/or Mn salts added to Promix growth medium a Replicate control plants b Indicates Ni concentrations significantly higher than means for the same plant in Table 2 During this time supplemental high-intensity sodium and incandescent lights capable of supplying 400 μmol m−2 s−1 supplemented sunlight if necessary. Daytime temperature was 24°C with cooling initiated at 27°C. Nighttime temperature was 18°C with cooling initiated at 21°C. Plants were grown in freely drained plastic pots with saucers to prevent loss of leachate, and watered with deionized water. The plants received standard fertilization (100 mg N, P2O5, K2O) once every two weeks. All plants grew normally with no signs of phytotoxicity. There were no significant differences in biomass, rate of growth, growth habit or leaf size among the various treatments. On 26 May (A. murale) and 12 June 2006 (A. corsicum) they were transplanted to 1,000 ml pots with the same Ni/Mn levels and grown until examined. Leaf harvest Two cm lengths of apical stem tip and leaves from various locations on the stem were harvested on 23 Mar. 2006 for initial Ni and Mn analysis. Leaves for cryogenic electron microscopy and standard energy dispersive Xray analysis (SEM/EDX) were harvested simultaneously. A second harvesting was done 27 July 2006 to establish whether leaf age (and related size) impacted Ni and Mn concentrations. This harvesting was done within a week of high-energy X-ray analysis. For each plant, four leaf subsets were sampled as follows: (1) youngest, smallest leaves from apical rosette; (2) young relatively small singular leaves from just below the apical rosette; (3) intermediate size and age leaves, representing the mode of leaves on the plant; (4) oldest largest leaves from stem areas distal to the apical rosette. Multiple leaves of each subset were collected from each plant at one sampling session. Because sampling took place in July, some of the plants were stressed. All the Alyssum hyperaccumulators we have grown are vigorous, fast-growing and disease-resistant; however, because they are native to Mediterranean climatic zones they become stressed under high heat/ humidity conditions. All plants we have grown thus far do relatively poorly in the Beltsville, MD greenhouse during July, August, and September, becoming prone to fungal infections and insect infestations, and dropping leaves (plants grown outdoors with good drainage and full sun are not affected). Symptoms are exacerbated in plants not allowed to hyperaccumulate Ni. Total metals analysis Harvested leaves were rinsed in deionized water to remove any adhering soil particles. Leaf samples for each treatment were dried for 24 h at 60°C, weighed, and ashed in a 480°C oven for 16 h. After cooling, the ash was digested with 2 ml concentrated HNO3 and heated to dryness. The sample was then dissolved in 10 ml 3 N HCl, filtered through Whatman #40 filter 38 paper and brought to volume in a 25 ml volumetric flask using 0.1 N HCl. For quality control, reagent blanks and an in-house Alyssum standard were included. Ni and Mn were determined by inductivelycoupled plasma atomic emission spectrometry using 40 mg L−1 yttrium as an internal standard in all samples and standard solutions. Scanning electron and standard X-Ray fluorescence spectrometry (SEM/EDX) For microanalysis bulk A. corsicum leaf samples of intermediate size (#3 as described above) were frozen in liquid nitrogen and fractured to yield complement cross-sections. A. corsicum has the highest trichome density of 12 Alyssum hyperaccumulator species that have been studied in detail, so we utilized it to maximize our chances of sectioning trichome bases. The trichomes are distributed equally over upper and lower epidermii (Fig. 1 a); however the physiology of the trichomes and adjacent cells differs between upper vs. lower epidermis. On the upper epidermis trichomes ascend from cup-like depressions formed by the adjacent cells. The cup-shaped feature is much less distinct on the lower epidermis but can still be recognized (Fig. S1; see supplemental information). The cupped structure allowed us to identify trichome bases easily (especially on the upper epidermis) even when the trichome itself had broken off. One half of each complement remained frozenhydrated and was etched and imaged with SEM at 2.0 kV. The other half was freeze-dried at −80°C (Pearse–Edwards tissue drier) and examined with SEM/EDX at 20 kV. Details of our procedure are given in Broadhurst et al. (2004a). For EDX analysis, we utilized only the four treatments with both Ni and Mn additions. Freeze-dried complement fracture leaf sections were individually mounted on standard aluminum stubs coated with silver adhesive paste. Samples were placed on edge to view the cross sections that correspond to the complement fracture planes. Before analysis a light coat of carbon was deposited on the samples at a pressure of 13 Pa followed by 20 nm of aluminum deposited at 1.3 10−3 Pa. In order to control excessive charging, samples were first analyzed as is and then some trichomes gently scraped off with a glass rod. Data in Table 2 include analyses both with and without trichomes, however the maps presented in Figs. 4 and S4. are Plant Soil (2009) 314:35–48 Fig. 1 a Alyssum corsicum cryogenic complement fracture showing cross section and lower epidermis. b Whole fresh leaf SXRF map for Ni40/Mn40 plant. Left panel shows Ni, Mn, Ca maps and the tricolor map. Right panel shows the correlation for Ni Kα vs. Mn Kα counts for a selected trichome (masked on left) and the correlation for the whole leaf area (inset graph). Due to the extensive network of trichomes, Ca signal covers nearly 100% of the image, so for imaging purposes a very high threshold of Ca signal intensity was set exclusively from samples with trichomes removed. Mapping was rendered imprecise without removing some trichomes due to motion of the sample over the long duration in the electron beam. We used an Amray 1820D scanning electron microscope (KLA Tencor Corp., AMRAY Division, Bedford, MA), an EDAX ECON 4 detector with an active area of 10 mm and an EDAX DX Prime analyzer (EDAX Inc, Mahwah, NJ). The samples were analyzed under the following conditions: 15– 20 kV accelerating voltage, 20° tilt, 12 mm working distance, 17.66° takeoff angle. Each spot location was counted for 100 live seconds. Beam spot was 1 to 10 μm depending on the application, but always less than a cell diameter when targeting a specific cell/ location. Semiquantitative results were corrected using ZAF algorithms. Every location that was analyzed was also digitally photographed with the AMRAY instrument. Elemental X-ray maps of Ni, Mn, Ca, Cl, K, Mg, P, S and Si signal were done for a few locations that afforded excellent cross sections of individual trichome bases. X-ray compositional analysis and mapping were accomplished with a windowless detector using EDAX Genesis software. Elemental mapping was done at a dwell time of 200 μs, a time constant of 50 μs, and resolutions of either 256 × 200 or 512 × 400 pixels. Synchrotron X-ray fluorescence spectrometry (SXRF) SXRF images of whole fresh leaves were acquired at beamline X-27A of the National Synchrotron Light Source at Brookhaven National Laboratory, Upton, NY. This beamline uses Kirkpatrick–Baez mirrors to produce a focused 10–15 μm spot of hard X-rays with tunable energy achieved via a Si(111) monochromator. For SXRF imaging, the incident energy was fixed at 10 keV to excite all target elements simultaneously. Live plants were transported to the synchrotron facility in their pots, and fresh leaves of size #2 were b Plant Soil (2009) 314:35–48 excised from plants immediately prior to analysis. Leaves were mounted onto Kapton® tape and attached to the sample stage with no further preparation. Specimens were rastered in the path of 39 the beam by an XY stage oriented 45° to the incident beam, and X-ray fluorescence was detected by a 13-element Ge detector (Canberra) positioned 90° to the beam. 40 Plant Soil (2009) 314:35–48 Table 2 Nickel and manganese concentrations in Alyssum murale ‘Kotodesh’ and Alyssum corsicum whole leaves, mg kg−1 dry weight from ICP−AES, and interaction between Mn and Ni Treatment A. murale A. corsicum Ni (mmol/kg) Mn (mmol/kg) Foliar Mn (mg/kg) Foliar Ni (mg/kg) Foliar Mn (mg/kg) Foliar Ni (mg/kg) 0 0 0 10 10 10 40 40 40 0 10 40 0 10 40 0 10 40 102e 408c 461c 198de 649b 710b 281d 424c 1,320a 22d 53d 48d 3,430c 3,640c 2,490c 11,200a 9,710ab 8,770b 82d 220bc 510a 103cd 318b 240b 112cd 566a NSa <10d 40d <10d 2,760c 4,520b 1,070d 8,300a 8,320a NS Means followed by the same letter are not significantly different at P<0.05 According to the Waller–Duncan K-ratio t test Entries are the mean metal concentration in four leaf type subsets from each plant sampled in the summer at time of SXRF analysis. Ni foliar levels were significantly negatively affected by both Mn soil additions and hot, humid growing conditions. Less than ten indicates concentration below detection limits. a Ni40/Mn40 plant remained at the University of Delaware so was unavailable for sampling. Elemental maps were typically collected from a 1 to 3 mm2 area using a step size of 20 μm and a dwell time of 500 ms. Smaller areas such as individual trichomes were imaged at ∼10 μm. Leaf areas larger than 3 mm2 were not imaged because of the time required. Manganese and Ni X-ray absorption near-edge structure (μ-XANES) spectra were collected at points of interest selected from the SXRF images, as detailed in Tappero et al. (2006). The monochromator was calibrated to the Mn (6539 eV) or Ni (8333 eV) K-edge energy with metallic foils, and spectra were collected from 200 eV below the absorption edge to k values of 9 A−1. Results Ni and Mn concentrations Nickel and Mn concentrations for the composite spring leaf sample are given in Table 1. Both A. murale and A. corsicum hyperaccumulated Ni and accumulated high levels of Mn when higher exogenous levels of these metals were provided; both species followed the same accumulation patterns. The mean values of the leaf subsets (Table 2) follow the same trend as the composite samples but the maximum Ni accumulation was significantly lower. Mn levels in the leaves uniformly increased over background as soil Mn levels increased. The back- ground Mn levels in the control A. murale samples were significantly higher than the corresponding A. corsicum controls; both controls were Promix hence Mn-sufficient. The highest Mn accumulation (924 mg kg−1), observed in A. corsicum Ni40/Mn40, was approximately ten times higher than control. For the Ni40 plants, leaf Ni concentrations decreased as Mn concentrations increased. At Mn40 levels neither species was able to accumulate as much Ni as Mn0 plants did (Tables 1, 2). Interestingly, for both species, the highest Mn concentrations were observed in the Ni40/Mn40 plants, not the Ni0/Mn40 plants—the reverse of the situation with Ni. For the Ni10 plants, Ni generally decreased as Mn increased with the exception that the Ni10/Mn10 plants had higher Ni than the Ni10/Mn0 plants. For the four leaf type subsets, there was no significant pattern in Ni and Mn accumulation with respect to size, age, or position on the stem. Our data indicate that leaves begin to hyperaccumulate Ni and accumulate Mn as soon as they emerge and continue to do so as they grow and age. Therefore both leaf sizes (#2, #3) that were X-ray analyzed can be considered representative of the plant as a whole. Overall, the composite samples accumulated nearly twice as much Ni as the leaf type subset mean. This almost certainly reflects sampling in March vs. late July, when the plants were stressed, as discussed in Materials and Methods. Plant Soil (2009) 314:35–48 There may be a tendency for leaf type 3 to contain the greatest amount of Ni but clearly identifying this pattern would require growing and analyzing multiple healthy, mature plants at each Ni/Mn addition level, which is beyond the present scope. Type 3 represents the mode of leaves on healthy plants, however Type 3 leaves may not be the most abundant on stressed plants. Stressed plants tend to drop lower leaves, sometimes retaining only the apical rosette. This is especially true for A. corsicum, which has relatively large, thick leaves and stems—hence many fewer leaves and stems occur on a given plant as compared to A. murale, A. pterocarpum, A. pintodasilva and other species. SXRF analysis The epidermis of each whole fresh leaf we examined showed the same basic pattern of Mn concentrations localized in distinct spots that are associated with trichome bases (Figs. 1b, S3). The stellate distributions of high Ca concentration are trichome rays, which are filled or covered with Ca carbonate and/or oxalate crystallites, and yield X-ray signals from Ca, Mg, C and O only. The entire leaf surface of A. corsicum is very densely packed with trichomes that form a layer 20–30 μm above the leaf epidermis (Figs. 1a, S1). The Ca X-ray fluorescence is so intense from this layer on flat-leaf images that it essentially masks underlying signals from other elements. Therefore we have shown representative Ca signal only from a few trichomes in Figs. 1 b and S3 in order that the Ni and Mn data remain visible. The typical intense Ca signal from the trichome rays can be seen along the leaf margin in these figures. Nickel and Mn distributions are spatially correlated but not homogenously distributed. Figure 1b (Ni40Mn40) shows somewhat reduced Ni signal intensity as compared to Fig. S3, (Ni10Mn10), in agreement with the whole leaf Ni data (Table 1). Linear areas of higher Ni concentration near the center of the leaf roughly correspond to epidermal cells overlying vascular tissue; however with this technique Ni signal is detectable across the entire leaf surface. Analysis of the XANES spectrum from a trichome area clearly identified Mn2+ as the predominant Mn oxidation state (Fig. 2). Similarly, the Ni XANES spectrum of the general leaf epidermis shows that Ni is present as Ni2+ (Fig. S2). 41 Semiquantitative SEM-EDX analysis The SEM-EDX analysis focused on a few locations with images no larger than 40 × 20 μm in order to better understand the generalized Ni and Mn distribution pattern described above. Further, the cryogenic complement fracture method cleanly shears cells, or removes discs of cell membrane so that cell interiors can be accessed and distinguished clearly from cell walls. Larger scale imaging and analysis is needed to identify the many locations where cellular/vacuolar contents are wholly or partially absent. When the vacuole is “plucked out” of an epidermal cell during sample preparation or handling, the resultant concavity typically retains a residue which clings to the inner cell wall. This residue can be moderately high in Ni (i.e. significantly above EDAX detection limits), thereby leading to the (incorrect) conclusion that Ni is sequestered in cell wall, yet this residual Ni concentration is far lower than that of the intact vacuole. Table 3 and Figs. 3 and S4 provide examples of this situation. We focused primarily on cleanly sectioned trichomes and the epidermal cells immediately adjacent to trichomes. Ni was generally concentrated in upper and lower epidermal cells (Table 3). Please note that data in Table 3 are semiquantitative only. Approximate weight percents are given solely to facilitate understanding of Ni and Mn localization patterns and should not be construed as defining hyperaccumulation levels. The achievement of hyperaccumulation is based on the ICP-AES data for dry leaves (Tables 1 and 2). Nickel and Mn were both strongly concentrated in trichome bases and lower pedicles, and in cells adjacent to the trichomes (Figs. 3 and 4). As observed in the whole leaf, Ni and Mn were co-located in the trichome bases and adjacent cells but the maximum Ni concentration observed in a location did not always correspond with the maximum Mn concentration. In particular, the upper parts of trichome bases tended to have high Ni without much Mn accumulation.. Figure 3 confirms in cross-section the ring-like distribution of Mn observed by Tappero et al. (2007). In general, trichomes on the epidermis which overlay the main vascular bundle were observed to have vacuoles strongly enriched in Ni. Mn was at or below detection limits in cells below the epidermal layer, in trichome rays, and in general epidermal cells that were not associated with a trichome. Manganese must be present in all chloroplasts, but the typical foliar 42 Plant Soil (2009) 314:35–48 Fig. 2 Top left panel shows tricolor Ni–Mn–Ca elemental map for whole fresh leaf from a Ni40/Mn40 plant. The inset box delineates transect (1) across a trichome near the leaf margin. The line spectra across the transect verifies the ring-like distribution of Mn and Ni in trichome bases. On the top right panel, the μ-XANES spectra of spot 1 are compared to Mn II and Mn IV standards, demonstrating that the spot 1 absorption edge is similar to the Mn II standard concentrations are low compared to the levels in the trichome base spots. Sulfur was strongly correlated with Ni in the Ni40 samples (Fig. 4). For each individual line spectrum we collected, high Ni signal was always associated with much greater than average S signal intensity. We found no additional correlations between Ni or Mn and K, Cl, P or Si. The central area of Fig. S4 has a concavity which corresponds to the projection of the tip of trichome base in the complement fracture. Some material from the trichome base has been retained, and in a smaller scale image would probably be identified as mesophyll. The Ni and S are signals are strongest in the intact upper epidermis, but Ni and S also appear somewhat dispersed. The Ni Lα signal is particularly strong since that area is not adjacent to Plant Soil (2009) 314:35–48 43 Table 3 Representative peak/background count ratios expressed as ZAF-corrected semiquantitative weight percents for Ni and Mn in trichome bases and adjacent cells from EDX point analyses (A. corsicum) Sample/Location Ni10/Mn10 ADJ C (3) TBa basal TBa mid TBa apical TBb apical Ni40/Mn10 ADJ C (3) TB (5) TB cell wall Ni10/Mn40 ADJ C (7) TBa basal TBa mid TBa apical TBb basal TBb mid TBb apical Ni40/Mn40 ADJ C (6) epidermal cella Mesophylla Mesophyllb TB (5) TB basal TB mid 1 TB mid 2 TB apical Ni wt% Mn wt% Comment 9.0±1 8.0 6.1 6.6 4.7 DL DL DL 2.0 2.1 high concentration area (HCA) HCA 9.0±1 8.9±2 1.9 DL DL DL 94% C and O; low metals 4.5±2 3.3 DL DL 9.0 DL DL 2.5±1 8.7 DL DL 12 1.4 DL 11±4 27 4.6 2.4 4.2±2 2.3 4.5 2.3 19 8±5 1.2 DL DL 1.9±0.5 DL 1.4 1.9 1.9 HCA HCA 2 cells away from TB listed below spongy, next to ADJ spongy, wide area scan whole TB scans HCA but contents mainly absent HCA but contents mainly absent above mid 1,” “ HCA, contents present In general mesophyll and vascular tissue yielded insignificant Ni and Mn counts so data are not reported. Values in parentheses indicate number of locations averaged. ADJ C -adjacent cell; TB-trichome base. When possible trichome bases were analyzed stepwise from the basal area upwards. DL values at or below 1% a General epidermal cells not directly associated with trichomes always had insignificant Mn counts and may or may not have significant Ni counts, depending on the Ni soil level. This cell had a very large intact vacuole. It may have been an adjacent cell for another trichome but it could not be determined. the trichomes, which can cause scattering of lower energy X-rays. We also observed that on occasion both Ca and Mg were strongly concentrated in the trichome rays in a ratio consistent with dolomite. Discussion Nickel and Mn concentration and distribution patterns in A. corsicum resulting from the factorial additions of Ni and Mn salts confirm previous observations of Alyssum species in non-Mn enriched soils. Previously we found that Mn and Zn levels were elevated in Alyssum leaves across a series of Ni additions (10– 90 mmol Ni kg−1) as compared to control and Nianalytical standard plants (Broadhurst et al. 2004a). Concentrations in duplicates of A. murale grown in Promix without Mn addition were as follows: for Ni10 plants: 10,900 and 9,470 mg Ni kg−1; 286 and 389 mg Mn kg−1. For Ni40 plants: 17,300 and 17,000 mg Ni kg−1; 279 and 223 mg Mn kg−1. Data for Mn0 treatments in this study were comparable. We first detected localized high concentrations of Mn in A. murale ‘Kotodesh’ plants grown in natural serpentine soil (Kukier and Chaney 2001; Broadhurst et al. 2004b). Leaves from these plants contained 8040 44 Plant Soil (2009) 314:35–48 spot Ni % Mn % 1 3 nd 2 7 2 3 16 9 4 23 2 5a 23 20 5b 29 22 5c 27 20 6 17 18 7 47 2 8 8 nd Fig. 3 a Trichome located on the lower epidermis in the vicinity of the main vascular bundle. b Close up of the trichome and adjacent cells. Semiquantitative oxygen-free Ni and Mn weight percentages for the points designated are listed. Unavoidable concave sample geometry resulted in low oxygen counts but did not affect metal signals. Note that locations 1 and 2 have the bulk of the cellular contents missing; point 4 is more representative of the trichome base Ni content overall. (nd not detected) mg Ni kg−1and 801 mg Mn kg−1. A. corsicum leaves from plants grown in the serpentine soil contained 7600 Ni mg Ni kg−1 and 289 mg Mn kg−1. Similarly, in the current study Mn levels in the control A. murale plants were significantly greater than the corresponding A. corsicum controls. However, both species concentrated Mn when it was provided at higher phytoavailable levels. Both Ni hyperaccumulation and Mn accumulation are initiated when leaves are young and continue at approximately the same rate as the leaf ages. The most important difference observed upon addition of high soil Mn was that Ni foliar concentrations decreased as Mn foliar concentrations increased, indicating that increasing soil Mn levels interfere with Ni uptake, translocation and/or storage. Further, the greatest Mn concentrations were observed in the Ni40/Mn40 plants, not the Ni0/Mn40 plants, indicating that Ni did not interfere with Mn accumulation and may in fact potentiate it. Therefore maximum Ni phytoextraction by Alyssum may be reduced in soils with high phytoavailable Mn. Low S may also decrease Ni phytoextraction. The positive correlation between Ni and S localization was strong, probably reflecting NiSO4 storage in epidermal vacuoles as suggested previously (Küpper et al. 2001; Broadhurst et al. 2004b). A similar correlation of Ni and S X-ray counts and localization patterns was observed in plants grown from hyperaccumulator populations of A. bracteatum and A. murale but not in control plants grown from a nonserpentine A. murale population (Asemaneh et al. 2006). The Ni2+ oxidation state for hyperaccumulated Ni in the leaf epidermis is consistent with NiSO4 storage but does not rule out other ligands. The strongly heterogenous Mn distribution pattern is characteristic of many plants, which exhibit high Mn “spots” on leaves when grown with high Mn supplied, as opposed to uniform tissue Mn elevation (Blamey et al. 1986; Marschner 1995; Iwasaki and Matsumura 1999). Manganese spots are generally described as a response to Mn toxicity, since they eventually become necrotic as Mn levels increase. Our Alyssum plants did not exhibit symptoms of Mn phytotoxicity yet maintained very tight control on the “spot” distribution pattern, with high Mn concentrations restricted to trichome bases and the cells immediately adjacent to trichomes. We observed very high localized Ni and Mn concentrations—sometimes over 20 dry wt%—only Plant Soil (2009) 314:35–48 45 Fig. 4 SEM guide photo of A. corsicum Ni40/Mn40 plant (a). Leaf cross section, lower epidermis; trichome base (trichome broken off) and adjacent cells in center of image. X-ray maps (20 KeV; Kα, β) show Ni (b), Mn (c) and S (d) distribution. Map overlay software showed nearly 100% correlation between Ni and S distribution within trichome bases and intact vacuoles inside epidermal cells associated with trichomes. As discussed previously, analyses must be undertaken carefully to ensure that residues on the cell wall are clearly distinguished from cell wall proper, since these residues may still contain a respectable 5 wt%. It is also imperative that quantitative whole leaf analyses back up semiquantitive fluorescence mapping, so that total leaf Ni concentrations are known to be >1,000 μg g−1. For example, the mean Ni dry wt% for 13 epidermal spots analyzed by EDX reported in Smart et al. (2007) is 0.29%, with 7 of the locations containing undetectable concentrations. Since it is clear that epidermal cells contain the bulk of the Ni in Alyssum hyperaccumulator species, the corresponding whole leaf concentrations cannot be within the realm of hyperaccumulation, thus may bear little relevance towards development of useful phytoremediation/phytomining technologies. Further, the standard ZAF matrix corrections on these highly nonuniform biolog- ical samples are not accurate enough that 0.29 wt% can be considered a significant result. To err on the conservative side, we do not report semiquantitative weight percentages under 1% as detections. The fresh whole leaf SXRF technique ensures that metals are not redistributed during preparation for analysis, so is an excellent check on other methods which require sample dehydration and/or fixation. Leaf folds and curls as observed herein and discussed in detail in Tappero et al. (2007) are the major caveat. Also using whole leaf SXRF and A. murale ‘Kotodesh,’ McNear et al. (2005) reported high Ni concentrations associated with vascular tissue which they explained as “entrainment during fixation as opposed to tissue loading.” We observed a pattern consistent with this although we did not have a whole leaf cross-section. Previous authors are in agreement that Ni is not stored in vascular tissue (Krämer et al. 1997; Küpper et al. 2001; Psaras et al. 2000; Kerkeb and Krämer 2003; Marmiroli et al. 2003; Broadhurst et al. 2004a, b; 46 Asemaneh et al. 2006; De La Fuente et al. 2007; Tappero et al. 2007) yet the whole leaf fresh SXRF data indicate a pattern of elevated Ni signal intensity that roughly correlates with the vascular system. An association of Ni with vascular tissue is logical given that the vascular system must transport Ni and Mn. The pattern of relatively higher Ni concentration associated with the vascular system may be a combination of preservation of Ni-rich fluids in transit as the leaf is harvested, and an elevated concentration in storage vacuoles in epidermal tissue/trichomes which overly the vascular bundle. Manganese concentration at the base of trichomes has also been observed in sunflower (Blamey et al. 1986), pumpkin (Iwasaki and Matsumura 1999) and cucumber (Horiguchi 1987). In these crop plants, trichome Mn storage increased and Mn phytoxicity decreased at a given Mn level when silicon was supplied to the plants. Apparently, Si facilitates the storage of Mn at the trichome base in a metabolically inactive form. In the case of Alyssum, localized high Mn concentrations are associated with trichomes at much lower whole leaf Mn levels than present in the other species—where Mn localization was observed under Mn-phytotoxic conditions. Further, in whole fresh Alyssum leaves Mn2+ is the predominant oxidation state, not the insoluble, oxidized Mn4+. These trichome-associated localization patterns contrast with the pattern observed in the Mn hyperaccumulators/strong accumulators Gossia bidwillii, Virotia neurophylla, Macadamia integrifolia and M. tetraphylla, where the highest Mn concentration was measured in palisade mesophyll (Fernando et al. 2006a,b). Further studies of the Ni–Mn interaction in Ni-hyperaccumulators using Mn concentrations approaching phytotoxicity to Alyssum will be necessary to understand the localization patterns. Higher plants with two functionally distinct vacuoles within a single cell have been reported by several research groups (Paris et al. 1996; Jauh et al. 1999; Epimashko et al. 2004; Epimashkoand Fischer-Schliebs 2006; Martinola et al. 2007). A model with two or more discrete vacuoles within an epidermal cell fits the observation that Ni and Mn were co-located in trichome bases but accumulated in separate areas as opposed to being interspersed. Our complete series of SEM photographs of frozen hydrated leaf cross sections does contain images which have a visible demarcation line across a trichome base or adjacent cell vacuole. Plant Soil (2009) 314:35–48 The present findings raise the possibility that Ni hyperaccumulation in Alyssum species originally developed from the Mn handling system. Most serpentine soils contain high levels of Mn in the form of oxides. The unexpected high Mn concentration in discrete regions at the base of trichomes has no apparent physiological basis, but it clearly occurs in Alyssum Ni hyperaccumulators (Küpper et al. 2001; Broadhurst et al. 2004b; McNear et al. 2005; Tappero et al. 2007). This localized high-concentration Mn2+ storage suggests the presence of a tonoplast Mn permease specific to trichome pedicles, while high epidermal cell vacuolar Ni storage similarly suggests the presence of a tonoplast Ni permease. Whether sulfate is also actively transported into these cells needs to be firmly established. Also unexplained is the mechanism whereby trichome cells push Ca and Mg into the trichome rays while storing Ni and Mn in the base. Yet Alyssum murale is also selective enough that it excretes (serpentine) geogenic Co exocellularly via hydathodes (Tappero et al. 2007), and virtually excludes geogenic Cr from uptake (Brooks 1987; Bani et al. 2007). De La Fuente et al. (2007) also observed significant Mg along with Ca in trichomes, suggesting that both these metals are present in an inorganic form in trichome nodules. It has been suggested that the serpentine-endemic Mnhyperaccumulators Alyxsia sp. and Austomyrtus bidwiilii accumulate Mn at the expense of Ca and Mg (Brooks et al. 1981; Bidwell et al. 2002), so the potential for interaction among all stored metals exists for hyperaccumulator species in general. These questions add to the remaining mysteries of hyperaccumulators and require scientific explanation if we are to obtain improved hyperaccumulator cultivars for practical remediation of contaminated soils. Acknowledgements We greatly appreciate the assistance of USDA-ARS personnel Carrie Green for ICP analyses and Christopher Pooley for computer graphics. References Asemaneh T, Ghaderian SM, Crawford SA, Marshall AT, Baker AJM (2006) Cellular and subcellular compartmentation of Ni in the Eurasian serpentine plants Alyssum bracteatum, Alyssum murale (Brassicaceae) and Cleome heratensis (Capparaceae). Planta 225:193–202 Baker AJM, McGrath SP, Reeves RD, Smith JAC (2000) Metal hyperaccumulator plants: a review of the ecology and physiology of a biochemical resource for phytoremedia- Plant Soil (2009) 314:35–48 tion of metal-polluted soils. In: Terry N, Banuelos G (eds) Phytoremediation of Contaminated Soil and Water. Lewis Publishers, Boca Raton, FL, pp 85–107 Bani A, Echevarria G, Sulçe S, Morel JL, Mullai A (2007) In-situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site (Albania). Plant Soil 293:79–89 Bidwell SJ, Woodrow IE, Batianoff GN, Sommer-Knudsen J (2002) Hyperaccumulation of manganese in the rainforest tree Austromyrtus bidwillii (Myrtaceae) from Queensland, Australia. Funct Plant Biol 26:899–905 Blamey FPC, Joyce DC, Edwards DG, Asher CJ (1986) Role of trichomes in sunflower tolerance to manganese toxicity. Plant Soil 91:171–180 Broadhurst CL, Chaney RL, Angle JA, Maugel TK, Erbe EF, Murphy CA (2004a) Simultaneous hyperaccumulation of nickel, manganese and calcium in Alyssum leaf trichomes. Environ Sci Technol 38:5797–5802 Broadhurst CL, Chaney RL, Angle JA, Erbe EF, Maugel TK (2004b) Nickel localization and response to increasing Ni soil levels in leaves of the Ni hyperaccumulator Alyssum murale. Plant Soil 265:225–242 Brooks RR (1987) Serpentine and its Vegetation: A Multidisciplinary Approach. Dioscorides Press, Portland, OR Brooks RR, Trow JM, Veillon J, Jaffré T (1981) Studies on manganese-hyperaccumulating Alyxia species from New Caledonia. Taxon 30:420–423 Chaney RL, Angle JS, Baker AJM, Li Y-M (1999) Method for phytomining of nickel, cobalt and other metals from soil. US Patent No. 5,944,872 issued Aug. 31, 1999. (continuation-inpart of US Patent 5,711,784 issued Jan. 27, 1998). Chaney RL, Angle JS, Broadhurst CL, Peters CA, Tappero RV, Sparks DL (2007) Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J Environ Qual 36:1429–1443 De La Fuente V, Rodríguez N, Díez-Garretas B, Rufo L, Asensi A, Amils R (2007) Nickel distribution in the hyperaccumulator Alyssum serpyllifolium Desf.spp. from the Iberian Peninsula. Plant Biosyst 141:170–180 Epimashko S, Fischer-Schliebs E (2006) Na+/H+-transporter, H+ pumps and an aquaporin in light and heavy tonoplast membranes from organic acid and NaCl accumulating vacuoles of the annual facultative CAM plant and halophyte Mesembryanthemum crystallinum L. Planta 224:944–951 Epimashko S, Meckel T, Fischer-Schliebs E, Luttge U, Theil G (2004) Two functionally different vacuoles for static and dynamic purposes in one plant mesophyll cell. Plant J 37:299–300 Fernando DR, Bakkaus EJ, Perrier N, Baker AJM, Woodrow IE, Batianoff GN et al (2006a) Manganese accumulation in the leaf mesophyll of four tree species: a PIXE/EDAX localization study. New Phytol 171:751–758 Fernando DR, Batianoff GN, Baker AJ, Woodrow IE (2006b) In vivo localization of manganese in the hyperaccumulator Gossia bidwillii (Benth.) N. Snow & Guymer (Myrtacea) by cryo-SEM/EDAX. Plant Cell Environ 29:1012–1020 Horiguchi T (1987) Mechanism of manganese toxicity and tolerance of plants. II. Deposition of oxidized manganese in plant tissues. Soil Sci Plant Nutr 33:595–606 Inamdar JA, Rao NV (1983) Light and scanning electron microscopic studies on trichomes of some Brassicaceae. Feddes Repert 94:183–190 47 Iwasaki K, Matsumura A (1999) Effect of silicon on alleviation of manganese toxicity in pumpkin (Curcubita moschata Duch cv. Shintosa). Soil Sci Plant Nutr 45:909–920 Jauh GY, Phillips TE, Rogers JC (1999) Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 11:1867–1882 Kerkeb J, Krämer U (2003) The role of free histidine in xylem loading of nickel in Alyssum lesbiacum and Brassica juncea. Plant Physiol 131:716–724 Krämer U, Grime GW, Smith JAC, Hawes CR, Baker AJM (1997) Micro-PIXE as a technique for studying nickel localization in leaves of the hyperaccumulator Alyssum lesbiacum. Nucl Instrum Methods Phys Res B 130:346–350 Kukier U, Chaney RL (2001) Amelioration of nickel phytotoxicity in muck and mineral soils. J Environ Qual 30:1949–1960 Küpper H, Lombi E, Zhao F-J, Wieshammer G, McGrath SP (2001) Cellular compartmentation of nickel in the hyperaccumulators Alyssum lesbiacum, Alyssum bertolonii and Thlaspi goesingense. J Exp Bot 52:2291–2300 Li Y-M, Chaney RL, Brewer E, Angle JS, Nelkin J (2003a) Phytoextraction of nickel and cobalt by hyperaccumulator Alyssum species grown on nickel-contaminated soils. Environ Sci Technol 37:1463–1468 Li Y-M, Chaney R, Brewer E, Rosenberg R, Angle JS, Baker AJM et al (2003b) Development of a technology for commercial phytoextraction of nickel: economic and technical considerations. Plant Soil 249:107–115 Marmiroli M, Maestri E, Gonelli C, Gabrielli R, Marmiroli N 2003 Dealing with Ni: comparison between a hyperaccumulator and a non-hyperaccumulator species of Alyssum on serpentine soils. Abstracts for the New Phytologist Symposium “Heavy Metals and Plants: from Ecosystems to Biomolecules”, 30 Sep to 1 Oct 2003 University of Pennsylvania, Philadelphia, PA. New Phytologist Trust, London, UK. Marschner H (1995) Mineral Nutrition of Higher Plants. Academic, London, UK Martinola E, Maeshima M, Neuhaus HE (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58:83–102 McNear D Jr, Peltier E, Everhart J, Chaney RL, Newville M, Rivers M et al (2005) Application of quantitative fluorescence and absorption-edge computed microtomography to image metal compartmentalization in Alyssum murale. Environ Sci Technol 39:2210–2218 Oran S (1996) Trichomes of the genus Alyssum L. (Crucifera) in Jordan. Webbia 50:237–245 Paris N, Stanley CM, Jones RL, Rogers JC (1996) Plant cells contain two functionally distinct vacuolar compartments. Cell 85:563–572 Psaras GK, Constantinidis TH, Cotsopoulos B, Maneta Y (2000) Relative abundance of nickel in the leaf epidermis of eight hyperaccumulators: evidence that the metal is excluded from both guard cells and trichomes. Ann Bot (Lond) 86:73–78 Reeves RD (1992) The hyperaccumulation of nickel by serpentine plants. In: Baker AJM et al (ed) The vegetation of ultramafic (serpentine) soils. Intercept, Andover, Hampshire, UK, pp 253–277 Robinson BH, Lombi E, Zhao FJ, McGrath SP (2003) Uptake and distribution of nickel and other metals in the hyperaccumulator Berkhya coddi. New Phytol 158:279–285 48 Smart KE, Kilburn MR, Salter CJ, Smith JAC, Grovenor CRM (2007) NanoSIMS and EPMA analysis of nickel localisation in leaves of the hyperaccumulator plant Alyssum lesbiacum. Int. J. Mass 260(Spec.):107–114 Tappero R, McNear D Jr, Gräfe M, Marcus MA, Sparks DL (2006) Elemental distributions, associations, and molecular speciation in plant material using synchrotron X-ray fluorescence (μ-SXRF) imaging and X-ray absorption Plant Soil (2009) 314:35–48 fine-structure (XAFS) spectroscopy. In: Luster J, Finlay R (eds) Handbook of methods used in rhizosphere research. Swiss Federal Research Institute, Birmensdorf, pp 209–21 Tappero R, Peltier E, Grafe M, Heidel K, Ginder-Vogel M, Livi KJT et al (2007) Hyperaccumulator Alyssum murale relies on a different metal storage mechanism for cobalt than for nickel. New Phytol 175:641–654