Document 10694258
advertisement

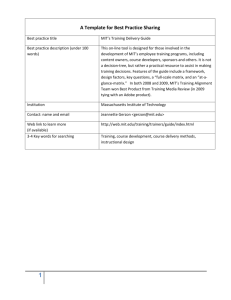
(Some) Numbers and Maths in Biology Jörn Dunkel E17-412 dunkel@math.mit.edu http://bionumbers.hms.harvard.edu/ dunkel@math.mit.edu Phylogenetic tree source: wiki dunkel@math.mit.edu DNA source: wiki • • http://ghr.nlm.nih.gov/handbook/basics/dna DNA contour length in bacteria: ~1.5mm Length of DNA in nucleus of mammals: ~ 2m dunkel@math.mit.edu DNA = biopolymer pair ~ 3m per cell ! ~ 10^14 cells/human ! > max. distance between Earth and Pluto (~50 AU = 7.5 x 10^12 m) dunkel@math.mit.edu DNA packaging in eukaryotes dunkel@math.mit.edu source: wiki mass dunkel@math.mit.edu source: wiki dunkel@math.mit.edu Prokaryotes http://www.sci.sdsu.edu/~smaloy/MicrobialGenetics/topics/chroms-genes-prots/genomes.html dunkel@math.mit.edu Typical length scales http://www2.estrellamountain.edu/faculty/farabee/BIOBK/biobookcell2.html dunkel@math.mit.edu Species estimates • estimated number of eukaryotic species on Earth: 8.7 million (Nature, 2011) • • • • undiscovered: 86% land spec. & 91%marine spec ~ 300,000 plant species prokaryotic biomass ~ eukaryotic biomass oldest known fossilized prokaryotes from 3.5 billion years ago dunkel@math.mit.edu Size-Complexity relation dunkel@math.mit.edu Unicellular organisms Algae Chlamydomonas reinhardtii (K. Drescher) Bacteria Caulobacter crescentus (Gitai lab, Princeton) Text ! size ~ 10µm doubling time ~ 5-8h size ~ 1µm doubling time ~ 2h Amoeba size ~ 1mm doubling time ~ 1d dunkel@math.mit.edu evolution from unicellular to multicellular ? dunkel@math.mit.edu Volvox carteri 200 ㎛ 10 ㎛ Chlamydomonas reinhardtii dunkel@math.mit.edu Volvox carteri somatic cell cilia 200 ㎛ daughter colony Drescher et al (2010) PRL dunkel@math.mit.edu how do organisms achieve locomotion ? dunkel@math.mit.edu lds Numbers in Biology Reynolds numbers number is dimensionless group that characterizes the ratio o fined as ⇥U L UL Re = = µ density of the medium the organism is moving through; µ is t ; is the kinematic viscosity; U is a characteristic velocity of stic length scale. When we discuss swimming biological organ eatures that are moving through water (or through a fluid with hose of water). This means that the material properties µ and ber is roughly determined by the size of the organism. e characteristic size of the organism and the characteristic sw rule-of-thumb, the characteristic locomotion velocity, U , in bi y U L/second e.g. for people L 1 m and we move at U 1 dunkel@math.mit.edu E.coli (non-tumbling HCB 437) Drescher, Dunkel, Ganguly, Cisneros, Goldstein (2011) PNAS dunkel@math.mit.edu labeled, non-tumbling E. lines coli due as they through a suspenexistence of closed stream to the swam presence of the wall. (H) The flow field of an E. coli “pu presence ofthe the wall.tracer (H) The flow field of an E.measured coli “pusher” decays much when ab dthe by subtracting best-fit dipole from field. The presence of faster, the flagella However, the force sionsince of fluorescent particles. far from it is partially cancelled by the the experimentally flowFor fieldmeasurements of its “puller” image. Results w of itswe “puller” image. d. field Atwalls, distances rfocused <6µ mon theadipole model overestimates the bacterial flow field. (E) Experimentally measured flow to t plane 50 µm from the top and bottom tancesurfaces 2 µm parallel to sample the wall. chamber, (F) Best fitand force-dipole model, (G) residual field. Notewhere the Bacterial fl cell body, th of the recorded ∼ 2 and terabytes of flow 4 (non-tumbling HCB e flowmovie fieldResults ofdata. an E. In coli “pusher” decays much faster, a bacterium swims close thecule surface, fortothe length of th theflow meas fi this data we identified ∼437) 10when rare events when achieved by fitting theByTo measured andminisbest-fit force d decays of cellsBacterial swam in the for > surfaces. 1.5 s. tracking labeled, no flowfocal fieldplane far from resolve the the atspeed variable location fluid tracers in each of the rare events, relating their position of the ced decays of the flow u with cule flow field created by individual bacteria, we tracked gfp- sion of fluor m surfaces. To resolve the minisfield (r >field 8 µm). and labeled, velocity to the position and orientation of the bacterium, disp non-tumbling E. coli as they swam through a suspenof the cell body (Fig. 1D) illustra walls, we fo dividual bacteria, we tracked gfpthe measured and best-fit force dipole field (Fig. 1C). The the specific fitting r and performing an ensemble average over all tracers, we reHowever, sion of fluorescent tracer particles. For measurements far from field displays the characteristic 1/1 dipole length ℓ =of decays of the flow speed u with distance r from thesurfaces center i asministhey the swam through aflow suspensolved time-averaged field in the E. coli swimming he measured walls, we focused on a plane 50 µm from the top and bottom value of F is consis movie data However, the force dipole flow sign oftothe cell (Fig. 1D) illustrate that flow down 0.1% of body the mean swimming speed V0 = 22 ±the 5 measured ticles. For measurements far from ckedplane gfpcell body surfaces of the sample chamber, and recorded terabytes ofresistive 2 ∼ 2their and force t µm/s. As E. coli rotate about their swimming direction, cells swam field displays the characteristic 1/r decay of a force dipole. measured flow to the side of the 4 suspenea 50 µmmovie fromdata. the top and bottom for the be le In this data we identified ∼ 10 rare events when note that in the flow field in three dimensions isbody, cylindrically fluid tracer However, the force dipole flow significantly overestimates the cell where the flow magnitu sber, fartime-averaged from achieved and recorded ∼ 2 terabytes of cells swam in the focal plane forall>components 1.5 s. Byoftracking thebehind the cent µm symmetric. Our measurements capture this measured flow to when the side of the cell body,ofand behind the 4 and velocit didentified bottom for the length the flagellar bund at variab fluid drag on the fla ∼ 10 rare events fluid tracers in each of the rare events, relating their position cylindrically symmetric flow, except the azimuthal flow due to cellof body, where the floworientation magnitude u(r) isof nearly constant and perform abytes of field (r fo > achieved by fitting two opposite and velocity to the position and of the bacterium, the rotation the cell about its body axis. The topology ne for > 1.5fors.theBy tracking the length of the flagellar bundle. The force dipole fitthe was solved the nts when speci the measured flow field (Fig. 1A) is the same as that of a and performing an ensemble average over all tracers, we reat variable locations along the swi are events, relating their position Bacterial flowdown field achieved by fitting two opposite force monopoles (Stokeslets) dipole len plane ckingforce the dipole flow (Fig. 1B), defined by solved the time-averaged flow field in the E. coli swimming field (r > 8 µm). From the best and orientation of the bacterium, dipole flow describ at variable locations along the swimming direction to the far value of E F position µm/s. As plane down to 0.1% of the mean swimming speed V = 22 ± 5 0 the specific fitting routines fit with good and accuracy h i e average over all tracers, we refield (r > 8 µm). From the best fit, which is insensitive to and resist A E. coli r direction, their time-averag ℓF swimming acterium, µm/s. As 2rotate about their ˆ thisµm approximation u(r)in= the 3(r̂.coli d) − 1 r̂, routines A= , r̂fitting = length , regions, [1 ]= we dipole ℓ 1.9 and dipo ow field E. swimming 2 the specific fitting and obtain the note that |r| 8πηdimensions |r| is cylindrically s, we retime-averaged flow field in three symmetric. a wall. Focusing 2 value of F is consistent with optic dipole length 1.9±µm and dipole force F = of 0.42 This µm behin mean swimming speed V0 ℓ==22 5 capture symmetric. Our measurements all components thispN. wimming and applying the s cylindricall and resistive force theory calculatio fluid drag of Fforce, is consistent with optical trapforce measurements [45] where F isvalue the dipole ℓ the distance separating the ut direction, their symmetric flow, except the azimuthal flow due to the = their 22 ±cylindrically 5swimming resulted in a slight rotatio note thatThe in the best fit, the cell d and resistive force theory calculations [46]. It is interesting to pair, η the viscosity the fluid, dˆ the orientation vector theof flow field struct the rotation ofisof the cell about itsunit body axis. topology ion, their three dimensions cylindrically the measur (swimming direction) the best bacterium, and rbehind the distance surfaces, thebfi note thatflow inofthe fit, 1A) theµm cell drag Stokeslet isfrom 0.1 the measured field (Fig. is the same as that oflocated aof the center the cell ndrically nts capture all components of this force dipole Drescher, Dunkel, Ganguly, Cisneros, Goldstein (2011) PNAS Bacterial vector relative to the center of the dipole. Yet there are some ity of a no-slip µm behind the center of thefluid cell body, reflectingbundle. the surf force dipole flow (Fig. 1B), defined by dunkel@math.mit.edu drag possibly on the flagellar nts of this E.coli , except flow bacterium. due to Fig. 1. Averagethe flow fieldazimuthal created by a single freely-swimming (A) Experimentally measured flow field far from a surface. Stream lines indicate local direction of flo dipole Bacterial motors movie: V. Kantsler ~20 parts 20 nm Berg (1999) Physics Today source: wiki Chen et al (2011) EMBO Journal dunkel@math.mit.edu Torque-speed relation 200 nm fluorescent bead attached to a flagellar motor 26 steps per revolution 30x slower than real time 2400 frames per second position resolution ~5 nm Berry group, Oxford dunkel@math.mit.edu Chlamydomonas alga 10 ㎛ ~ 50 beats / sec Goldstein et al (2011) PRL 10 ㎛ speed ~100 μm/s dunkel@math.mit.edu Chlamy Merchant et al (2007) Science dunkel@math.mit.edu Sperm near surfaces A C B Fig. 1. Surface scattering of bull spermatozoa is governed by ciliary contact interactions, as evident from the scattering sequences of individual cells at two temperature values: (A) T = 10 °C and (B) T = 29 °C. The background has been subtracted from the micrographs to enhance the visibility of the cilia. The cyanPolin, Goldstein PNAS colored line indicates the corner-shapedKantsler, boundaryDunkel, of the microfluidic channels(2012) (see Movies S1 and S2 for raw imaging data). The horizontal dotted line in the last Surface + shear flow ! Kantsler et al 2014 (submitted) Amoeba Eukaryotic motors Sketch: dynein molecule carrying cargo down a microtubule http://www.plantphysiol.org/content/127/4/1500/F4.expansion.html Yildiz lab, Berkeley dunkel@math.mit.edu Molecular ncisco, San csf.edu Downloaded fro Downloaded from www.sciencemag.org on October h among ach head nd-overernately nd stays her head contrast, th of the ce as the diffusion nd to the tracking r probes) Walking modes dot) on the lever arm in the hand-over-hand model (left) and the inchworm model (right). The FIONA assay Myosin V: Walking or inchworming? Predicted movement for the heads and a dye molecule label (green The author is in the Department of Cellular and Molecular has revealed that myosin V, along with kinesin and VI, walksmodel hand-over-hand. dot) on the lever armmyosin in the hand-over-hand (left) and the inchworm model (right). The FIONA assay Pharmacology, University of California at San Francisco, San Francisco, CA 94107, USA. E-mail: yildiz@cmp.ucsf.edu 792 2006 0 FEBRUARY VOL 311 has revealed that myosin V, along with kinesin and myosin VI, walks hand-over-hand. 10 FEBRUARY 2006 VOL 311 SCIENCE www.sciencemag.org SCIENCE www.sciencemag.org Published by AAAS CREDIT: P. HUEY/SCIENCE CREDIT: P. HUEY/SCIENCE another e motor The bias e power hes to the irection. t is a fungo transntensive ovement tional change in the forward head (head 1) and mining the center of its emis- are pleased to present the prize- point of the myosin. The trajecFIONA, tracked of organic site. Again, the bylabeled headtoryalternately moved thisUsing conformation pulls the Irear head (headthe 2) movement sion pattern. However, of moving spots created winning essay Ahmet Yildiz, forward, while head 1 stays fixed on the track. dyes are not very bright and the a regional winner for North America three classes of steps. I observed the motor proteins myosin V, kinesin, and twice as far as the stalk moved and stopped as In the next step, head 2 stays fixed and pulls signal disappears quickly by who is the Grand Prize winner of 74-or 0-nm displacements for myosin VI,Alternatively, which were a single the other head moved Cy3head 1 forward. in the labeled inchworm with permanent photobleaching. dye on the first IQ domain, alterthe Young Scientist Award. (7). Unexpectedly, model (2) only the forward head catalyzes ATP This limited previous singlenating 52-and 23-nm steps for dye in the head region as follows. calmodulin showed significant flexibility when and always leads while the other head follows molecule tracking experiments to a precision of dye on the fifth IQ domain and alternating 42-and (seeMyosin figure below). around 30 nm (4). I have extended photostabil33-nm steps for dye on the immobile sixth IQ domainin (5) V. Bifunctional rhodamine (Br)–labelit hadtheATP bound, whereas it was In both of these mechanisms, the motor ity and brightness of single organic dyes 20 times (see figure below, left). needs two heads to be able to stay on the track by effectively deoxygenating the assay solution Kinesin. A human kinesin was specifically as it moves and its step size depends on the and using reducing agents, and I have achieved labeled on the head region with a single Cy3 length of the legs. However, myosin VI with 1.5-nm localization and collected 1.4 million pho- molecule. As the stalk took 8-nm steps, the short legs (8 nm) was observed to take the same tons from single organic dyes. The technique, head was observed to take alternating 16-nm long steps (30 nm) as myosin V. Moreover, a sin- named fluorescence imaging with one-nanome- and 0-nm steps (6). gle-headed processive motor has suggested that ter accuracy (FIONA), has improved spatial resoluMyosin VI. Myosin VI was labeled with a two heads are not necessary for processive tion in single molecule fluorescence by ~20-fold. single Cy3 molecule on a calmodulin-binding motion. These observations lead to another Using FIONA, I tracked the movement of site. Again, the labeled head alternately moved mechanism: biased diffusion of the motor the motor proteins myosin V, kinesin, and twice as far as the stalk moved and stopped as along the actin/microtubule lattice (3). The bias myosin VI, which were labeled with a single the other head moved (7). Unexpectedly, Cy3is provided by the initial push of the power dye in the head region as follows. calmodulin showed significant flexibility when stroke, and the motor most likely attaches to the Myosin V. Bifunctional rhodamine (Br)–label- it had ATP bound, whereas it was immobile in next binding site in the forward direction. Understanding motor protein movement is a fundamental step in understanding how cargo transport works within a cell, but despite intensive research, the mechanism underlying movement remained highly controversial. The most direct way to distinguish among these models is to measure how much each head moves when the motor walks. The hand-overhand model predicts that a head alternately moves twice the stalk displacement and stays stationary in the next step while the other head takes a step (see figure, left panel). In contrast, the inchworm model predicts that both of the heads move forward the same distance as the stalk (see figure, right panel). The diffusion model states that heads randomly bind to the track. Current nanometer-precision tracking techniquesV: (optical traps and probes) Myosin Walking orcantilever inchworming? Predicted movement for the heads and a dye molecule label (green dunkel@math.mit.edu 0). Based showed oncluded for kinen asym- chnique, Accuraking the accuracy NA, the step is rescence a totalope. The is a dif280 nm, ponds to ted with plied the lks in a alternatcements, (11). s experiwith a ch head Fig. 1B) d as the e immoent conglutamic cond homer with ines and 43C and B). Subthe hoof fluo- (13). The dye’s position was monitored as the kinesin moved on microtubules that were immobilized on a coverslip (13). Three different conposition time. aHowever, if the observed structs versus were used: homodimer with glutamic 17-nm steps arise from the convolution of twohoacid mutated to cysteine (E215C), a second sequential 17 nm, nm. . .), thenwith a modimersteps with (i.e., T324C, and 0a heterodimer dwell-time of the number of steps one head histogram lacking solvent-exposed cysteines and versus step-time duration will be the convolution the other head containing cysteines at S43C and of T324C, two exponential (11).(Fig. This1B). yields which areprocesses 2 nm apart Sub2 exp(–kt), thestoichiometric dwell time probability, P(t ) $ tk labeling was used for the howhich is zero at t $ 0, quantal rises initially, andofthen modimers, and single bleaching fluofalls, when k is the stepping rate constant. rescence confirmed that only a single dye In was contrast, if the 17-nm steps arise from a single present on each kinesin analyzed (fig. S1B). The process, then the dwell-time histogram would heterodimer was labeled with an excess ofbe dye and both single- and double-quantal bleaching was observed (13). In the absence of ATP, kinesins were stationary. In the presence of 340 nM ATP, discrete steps were observed for the three different kinesin constructs (Fig. 2). A total of 354 steps from 35 kinesins were observed. We typically collected 4000 photons per 0.33-s image. Traces from relatively bright kinesins ("5000 photons per image) are shown in Fig. 2; a histogram of 143 steps from 26 molecules is shown in Fig. 3A. The precision of step-size determination was 1.5 to 3 nm, based on measurement of the distance between the average positions of the PSF centers before and after a step (11, 14). The average step size derived from the step-size histogram (Fig. 3A) is 17.3 # 3.3 nm. We did not observe 8.3-nm steps or odd multiples of 8.3 nm. These data therefore strongly support a hand-over-hand mechanism and not an inchworm mechanism. The hand-over-hand mechanism predicts that these 17-nm steps alternate with 0-nm steps, which are not directly observable in a graph of REPORTS expected to yield an exponential decay (the Poisson-distributed rate). The dwell-time histogram of 347 steps for E215C and T324C (Fig. 3B) is well fit by the above convolution function (with k $ 1.14 # 0.03 steps per s), and not by the single-step decaying function. The rise near t $ 0 is not due to instrument artifacts: An exponential process for myosin V stepping (with dyes located to show every step) at very similar rates yields the expected monotonic decay with the same instrument (11). We also have immo- tional human kinesin, were mutated to cysteines for fluorescent dye labeling as described in the text. The bound nucleotide (adenosine diphosphate) is shown as a space-filling model in cyan. This figure was made with MolMol (22). Kinesin walks hand-over-hand Fig. 1. (A) Examples of two alternative classes of mechanisms for processive movement by kinesin. The hand-over-hand model (left) predicts that a dye on the head of kinesin will move alternately 16.6 nm, 0 nm, 16.6 nm, whereas the inchworm mechanism (right) predicts uniform 8.3-nm steps. The inchworm model was adapted with slight modification from (9). (B) The positions of S43 (red), E215 (green), and T324 (blue) on the rat kinesin crystal structure [from (6), Protein Data Base 2KIN]. These residues, whose numbers correspond to conventional human kinesin, were mutated to cysteines for fluorescent dye labeling as described in the Fig. 2. Position versus time for kinesin motility. The blue and green traces are from E215C nucleotide homodimer text. kinesin;The the bound red trace, from the heterodimer S43C-T324C kinesin. The numbers (adenosine is correspond to the step sizediphosphate) # %&. The uncertainties were calculated as described (11). Red lines shownpositions as a space-filling in between steps (plateau) and when the step occurs represent average of each model duration cyan. This figure was made with (jumps) based on data analysis. MolMol (22). Yildiz et al (2005) Science www.sciencemag.org SCIENCE VOL 303 30 JANUARY 2004 dunkel@math.mit.edu 67 Intracellular transport Chara corralina http://damtp.cam.ac.uk/user/gold/movies.html dunkel@math.mit.edu wiki dunkel@math.mit.edu Actin-Myosin F-Actin Myosin helical filament myosin-II myosin-V dunkel@math.mit.edu our lecture course: ! generic models of micro-motors dunkel@math.mit.edu Polymers & filaments (D=1) Dogic Lab, Brandeis Drosophila oocyte Physical parameters (e.g. bending rigidity) from fluctuation analysis Goldstein lab, PNAS 2012 dunkel@math.mit.edu Actin in 2D F-Actin helical filament Dogic Lab (Brandeis) dunkel@math.mit.edu Actin in flow PRL 108, 038103 (2012) PHYSICAL REVIEW LETTERS FIG. 1 (color online). Experimental setup. (a) Microfluidic cross-flow geometry controlled by a pressure differenc and outlet branches. (b) Close-up of the velocity field near the stagnation point, showing a typical actin filament. (c of an actin filament and definition of geometric quantities used in the analysis. Kantsler & Goldstein (2012) PRL were stored at !80 " C. Polymerization to form filamentous actin (F-actin) was achieved by addition of 1=10th volume of eigenfunctions W ðnÞ (and eigenvalues & conditions Wxxdunkel@math.mit.edu ð)L=2Þ ¼ Wxxx ð)L=2Þ ¼ our lecture course: ! • polymer models • how to relate fluctuations to mechanical properties dunkel@math.mit.edu Cell membranes (D=2) http://www.sbmp-itn.eu/sbmps/research_method/ source: wiki dunkel@math.mit.edu Cell membranes (D=2) Illustration by J.P. Cartailler. Copyright 2007, Symmation LLC. transport: stochastic escape problems shape: differential geometry red blood cells affected by sickle-cell disease source: wiki dunkel@math.mit.edu Blood cells: shape & function source: wiki red blood cells affected by sicklecell disease http://learn.genetics.utah.edu/ dunkel@math.mit.edu Optical tweezer source: wiki http://www.nature.com/ncomms/journal/v4/n4/extref/ncomms2786-s1.swf dunkel@math.mit.edu Dynamics of a vesicle in general flow viscosities of the inner and outer fluids. Another analytical microfluidic 4-roll mill device (23,24) manufactured in silicone approach based on a quasi-spherical vesicle approximated by a elastomer by soft lithography (Fig. 1). Particle tracking veloci1 J.spherical Deschamps, V. Kantsler, Segre, and V. Steinberg harmonics expansion E. used a perturbation scheme metry (PTV) measurements show fair agreement with numerical around the Lamb solution of the Stokes flow near a spherical Department of Physics Complex Systems, Weizmann Institute body in external shearof flow (1). Further refinement of the modelof Science, Rehovot, 76100 Israel resulted in dynamic equations for vesicle shape and inclination Author contributions: V.K. and V.S. designed research; J.D. performed research; J.D., V.K., Edited Harry Swinney,rather University of Texas at motion Austin, and Austin, andand approved May 2009 (received for review March 11, 2009) V.S. analyzed data;6, and V.S. wrote the paper. angle by (3,4) and L. described well both the TT the TX, E.S., The authors declare no conflict of interest. transition line "c(!), as verified by the recent experiments An approach to quantitatively study vesicle dynamics as well as stronger vesicle shape deformations (3,17). Moreover, a new 1 Direct Submission. (16–18). However, theV. recent experimental key finding of a new This article is a PNAS J. Deschamps, Kantsler, E. Segre, and V. Steinberg biologically-related micro-objects in a fluid flow,(TR), whichled is based on correspondence aspectshould is be the dependence on $̇ of the separate regions of 1To whom type of unsteady motion, which we dubbed trembling addressed. E-mail: victor.steinberg@weizmann.ac.il. to reconsider bothof theoretical models (17). TRadiffers from TU the combination a dynamical trap and control parameter, the existence of TR and TU (17). Precisely these features changed This article contains supporting information online at www.pnas.org/cgi/content/full/ Department of Complex Systems, Weizmann Institute of Science, Rehovot, 76100 Israel by oscillations in Physics 0902657106/DCSupplemental. % of to lessof than !/2 (rather 2!) and by ratio of the vorticity the strain rate, isthan suggested. The flow is the idea of vesicle dynamics as smooth and shape-preserved Dynamics of a vesicle in general flow A continuously varied betweenUniversity rotational, shearing, and elongaand called for2009 an (received adequatefor theoretical description. Edited by Harry L. Swinney, of Texas at Austin, Austin, TX,motion and approved May 6, review March 11, 2009) 11444 –11447 ! PNAS ! July 14, 2009 mill ! vol.device, 106 ! no. 28 dynamical trap, that www.pnas.org"cgi"doi"10.1073"pnas.0902657106 tional in a microfluidic 4-roll the Several theoretical models were suggested to describe the dystates in shear flow,shape their regions of existence, and Moreover, a new allows scanning to of quantitatively the entire phasestudy diagram of motions, i.e., as namics An approach vesicle dynamics well asof all 3stronger vesicle deformations (3,17). transitions have verified recently, 1 of separate regions of tank-treading (TT), tumbling (TU), andin trembling (TR), which using ais based biologically-related micro-objects a fluid flow, on (5,7,13). aspectAsiswethe dependence on only $̇ ofthethe them presented in ref. 5 describes adequately the experimental single vesicle even at ! " #in/#out " 1, where #in and #out are the B combination of a dynamical trap and a control parameter, the existence of ofTR TUwhich (17).isPrecisely these features changed data the (20). The main result thisand model, based on the viscosities of the inner and outer fluids. This cannot be achieved in ratioshear of the the strain rate,TTisand suggested. flow is theof !# idea vesicle as smooth #1, of second orderdynamics spherical harmonics, andand shape-preserved pure flow,vorticity where theto transition between either TU orThe approximation continuously varied rotational, shearing, and elongamotion and called forsolution, an adequate theoretical description. neglecting thermal noise, is a self-similar which reduces As a result, it is found that the vesicle TR is attained only at !>1.between the number of the dimensionless control parameters to just 2: S to describe the dydynamical states in a general are presented by the phase diagram tional in a microfluidic 4-roll mill device, the dynamical trap, that Several theoretical models were suggested 3 $ $ $ / 3&! and %' 4(1 & 23 30! , where & ' 7!$̇# in a space scanning of only 2 dimensionless control parameters. The findings of all 3 states in"/32) shear!/ flow, their regions of existence, and allows of the entire phase diagram of motions, i.e.,outR namics is the bending elasticity [taken further as & " 25 kBT'10(12 erg are in semiquantitative accord with the recent theory made for a transitions (5,7,13). As we have verified recently, only the 1 of tank-treading (TT), tumbling (TU), and trembling (TR), using a (21)]. The phase diagram of the vesicle dynamical states is quasi-spherical vesicle, although vesicles with large deviations them presentedbyinthe ref. 5 describes adequately the experimental " #in/experimentally. #out " 1, where #out 2-dimensional, are the single vesicleshape evenwere at !studied in andof parameterized variables (S,%), and indefrom spherical The# physics data (20). The main result of this model, which is based on the viscosities of the inner and outer fluids. This cannot be achieved in of other geometrical parameters. To scan all 3 regimes pendent TR is also uncovered. of TU motion to trace transitions among in aorder shear spherical flow, approximation of !# #1, them second harmonics, and pure shear flow, where the transition between TT and either or and $̇ and " , that is change the viscosities of one should vary both neglecting thermal noise, is a self-similar solution, which reduces !>1. As result, itremains is found TR isnderstanding attained only the at rheology of abiofluids a that great the vesicle Fig. 1. (A) Schematic of the microfluidic 4-roll mill device; Q1 and Q2 are the flow discharges, whose ratio defines the flow type. The flow is driven by gravity, inner and outer which is an impossible task to realize on and the ratio between the pressure drop P0 and the pressure difference between 2challenge, inlets #P determines Q1/Q (B) (1 ! !/s)/(1% !/s) as function of the reduced whose relies, inapresented a large part, onthe detailed thefluids, number of the dimensionless control parameters to just 2: S dynamical states in2.progress af $general are by phase diagram an individual vesicle. Because of topology of the phase diagram pressure drop (1- #P/P0). Inset: s as a function of (1 ! #P/P0). Large filled squares, P0 $ P; open squares, P0 $ 4/3P; small filled circles, P0 $ 5/3P, open circles, P0 $ 3/ $ studies of the dynamics of a single cell. Vesicles are a model $!/$30!, where & !$̇#outRpossibility 3&! and 4(1vesicle & 23"is/32) ' 7remaining 2P (in our specific configuration P&750 Pa). The solid line is the 3D FEMin simulation of theof flow. Experimental imperfections due to soft lithography lead to a space only 2 dimensionless control parameters. The findings (5,20), the only with%a ' single to observable quantitative discrepancy with simulations. system used to study the dynamic behavior of biological cells, (12 is the bending elasticity [taken erg are in semiquantitative accord with the recent theory made fortransitions a scan from TU to TR by varying $̇. further as & " 25 kBT'10 similar in some respects to red blood cells, and their dynamics in This limitation overcome a general of flow, the dynamical states is (21)].is The phasein diagram thewhere vesicle quasi-spherical vesicle, although vesicles with large shear flow been the subject theoretical (1–8), deviations simulations and high flow uniformity (Fig. 2 and SI Appendix). The have experiments were performedofinintensive the following way. A velocity gradient can be written as ' V " s & ( ) , where sik i k ik ikj the j 2-dimensional, parameterized by variables (S,%), and indefromvesicle spherical shape studied experimentally. The physics of was The accessible range of s was [0.05–0.32] s!1 and that of !/s numerical with given R andexperimental #,were measured initially in situ in the same (9–13), and (14–18) investigations. is the symmetric strain tensor, ) the vorticity vector, and s " j ! /s and s in the 1.18–9.23]. To reduce error bars on S and ", the geometricalA vesicle device, was followed at a prescribed value of pendent of other geometrical parameters. To scan all 3 regimes is a droplet of viscous fluid encapsulated by a TR isobservation also uncovered. 2 $tr(sik window. A feedback in the flow velocity was used to parameters R and # of each vesicle were measured in situ from )/2 the strain rate. The corresponding control parameters phospholipid bilayer membrane suspended in a fluid of either motion and transitions among hold a vesicle in the field of view for up to 10 TU/TR periods. a 3D reconstruction of its shape (20) in TT motion at low S, with for vesicles inofgeneral flow (5)to aretrace S '14 !s#outR3/3$ 3&! andthem in a shear flow, the same or different viscosity as the inner one. Both the volume To explore the whole space of parameters (S, "), vesicles with mean errors of 1.7% on R and of 6.1% on # within the range [0.3, $̇ and ", we that is change the viscosities of one vary $!()/s)/ $30both % " 4 (1 & 23 "/32)should !. In this paper, report the nderstanding of biofluids various values of R and # the wererheology loaded and individually observed, 2.5]. Since only vesicles with " $ 1 were used in this work, there and the surface area ofthe vesicle are conserved. The remains former a great inner and outer fluids, which is an impossible task to realize on phase diagram in such general flow. This approach uses an !/s was varied in steps progress during the experiment by changing s no error contribution from this parameter (20). Errors from and challenge, whose relies, in atolarge part, on detailed means that the vesicle membrane is considered be imperme#P, which is the pressure difference between 2 inlets (see Fig. 1). non-uniformity of the velocity field were below 2%; that cumuadditional control parameter vesicle. )/s, whichBecause is fixed toof unity in shearof the phase diagram an individual topology at time scale of of the experiment, studies ofwayon the dynamics a(S, single cell. and Vesicles are a flow model In least this thethe space of parameters ") was populated with the latter ates to overall mean errors of 8.7% on S and of 2% on ".able, (s " ) " $̇ /2), to study vesicle dynamics [it was suggested (5,20), the only remaining possibility with a single vesicle is to means the membrane dilatation is neglected since is 2D systemthat used to study the dynamic behavior ofitbiological cells, first by G. I. Taylor to study emulsions in a 4-roll mill (22)]. The scan transitions from TU to TR by varying $̇. fluid (1,2). theoretical, and computational efforts similar inExperimental, some respects to red blood cells, and their dynamics ratio in can be easily varied continuously in the experiment, during the last decade led to the observation and characterizaThis from limitation is overcome in from a general flow, where the TT to either TU or TR and TU shear beendynamics the subject of intensive theoreticalevidencing (1–8), transitions tion of 3flow stateshave in vesicle in shear flow. The existence velocity cangiven be written as 'i"V. k The " sik & (ikj)j, where sik to TR on the same gradient vesicle with R, !, and numerical and experimental (14–18) of the first 2,(9–13), tank-treading (TT) and tumbling (TU),investigations. and the experimentalispath the phase diagram depends the the across symmetric strain tensor, )j theonvorticity vector, and s " A vesicle is athem droplet of viscous fluid by a transition between were already predicted by aencapsulated phenom2way )/s and s are varied. The possibility to initial state and the $ tr(sik)/2 the strain rate. The corresponding control parameters enological modelbilayer of Keller and Skalak (19) and its phospholipid membrane suspended in afurther fluid ofobserve either all dynamical statesinwith the same vesicle, evenSfor " "!s# R3/3$3&! and for vesicles general flow (5) are '14 out extensions (2,11,12). Twoviscosity control parameters, theone. excess areathe volume the same or different as the inner Both 1 used in the current experiment, $ complements the previous 2-4! and the viscosity contrast " " # /# $ % the " 4shear (1 & 23dynamics "/32) !( )/s)/ 30 !.the Inother this paper, we report the ! " A/R out, determine based on flow (2–4,7–20). On and the surface area of the vesicle arein conserved. The views former the transition line "c(!) between TT and TU, which is indepenphase diagram such flow. This approach uses an hand, the experimental approachin used heregeneral will be advantageous means that therate vesicle membrane is considered to be imperme$̇ in the approximation of a fixed vesicle dent of the shear additional control parameter ) /s, which is fixed to unity in shear study the dynamics of other flexible microobjects, including able, with at least on the time scale of% the and theto latter withexperiment, respect to the flow shape, the vesicle inclination angle biological membranes and cells, in flow. flow (s " ) red " $̇blood /2), to study vesicle dynamics [it was suggested direction as thethe onlymembrane dynamical variable (2,9,10,16–19). Here, R it is 2D means that dilatation is neglected since first by G. I. Taylor to study emulsions in a 4-roll mill (22)]. The isfluid the effective vesicle radius, theoretical, related to theand volume via V " Results and Discussion (1,2). Experimental, computational efforts ratio can be easily varied continuously in the experiment, 4/3!R3, A is the vesicle surface area, and #in and #out are the Measurements of the vesicle dynamics were conducted in a during the last decade led to the observation and characterizaviscosities of the inner and outer fluids. Another analytical transitions from TT to either TU or TR and from TU microfluidic evidencing 4-roll mill device (23,24) manufactured in silicone tion of 3based states vesicle dynamics in approximated shear flow. The approach onin a quasi-spherical vesicle by a existence on the(Fig. same vesicletracking with given elastomer byto softTR lithography 1). Particle veloci- R, !, and ". The of the first 2, tank-treading (TT)a and tumbling (TU), and the spherical harmonics expansion used perturbation scheme metry (PTV)experimental measurements show agreement numerical pathfairacross the with phase diagram depends on the transition between them were already by a phenomaround the Lamb solution of the Stokes flow predicted near a spherical U APPLIED PHYSICAL SCIENCES U dunkel@math.mit.edu Volvox inversion http://www.damtp.cam.ac.uk/user/gold/movies.html dunkel@math.mit.edu our lecture course: ! • ‘differential geometry’ of membranes dunkel@math.mit.edu Stationary patterns dunkel@math.mit.edu Turing model A. M. Turing. The chemical basis of morphogenesis. Phil. Trans. Royal Soc. London. B 327, 37–72 (1952) dunkel@math.mit.edu wiki The matching of zebrafish stripe formation and a Turing model Kondo S, & Miura T (2010). Reaction-diffusion model as a framework for understanding biological pattern formation. Science, 329 (5999), 1616-20 dunkel@math.mit.edu odel equations must be invariant under ⇤ ⇤ ⇤, implying that U (⇤) = in Equation (2). Intuitively, the transformation ⇤ ⇤ ⇤ sider the simplest isotropic fourth-order model for at a the non-conserved or the observer position midplane of thescalar film (watchin watching it from below). scalar order-parameter ⇤(t, x),2dgiven by Swift-Hohenberg model The situation can be rather different, however, if 2 ⇧t ⇤ = F (⇤) + 0 ⇤ ⇤,microorganisms close to a liquid-solid interface, such(1) as th 2 2 slide (Figure 2). In thi cells in the2 vicinity of a 22glass ⇤ ⇥ = U (⇥) + ⇥ ⇥ (⇥ ) t 0 and ⇤ =2 ⌅ is⇥the d-dimensional ⇧t = ⇧/⇧t denotes the time derivative, trajectory of a swimming cell can exhibit a preferred han an. The force F is derived from a Landau-potental U (⇤) example, the bacteria Escherichia coli [40] and Cauloba Scalar field theory F = ⇧U , ⇧⇤ U (⇤) = b the c 4 components of the Levi-Civita tensor, ⌃a ij 2denotes 3 Cartesian ⇤ + ⇤convention + ⇤for, equal indices throughout. (2) a summation 2 3 4 derivative terms on the rhs. of Equation (1) can also be obtained by variational s from a suitably defined energy functional. In the context of active suspensions, d, for example, quantify local energy fluctuations, local alignment, phase ces, or vorticity. We will assume throughout that the system is confined to spatial domain ⇥ ⇥ Rd of volume ⇤ |⇥| = dd x, (3) g with periodic boundary conditions in simulations. r completeness, one should note that in the case of a conserved order-parameter the field equations would either have to take the current-form ⇧ ⌅ = ⌅ · J(⌅) Active patterns PRL (2013) B. subtilis tracer bright field fluorescence 3D bacterial suspension bright field fluorescence PRL (2013) 3D suspension Experiment: quasi-2D slice PRL (2013) Theory: 2D slice Vector field theory incompressibility E=( 0 2⇥ 2 )(⇥ v + ⇥v ) † † Active nematics Dogic lab (Brandeis) Nature 2012 autonomous motility, which are not observed in their passive ana- relative po 1 77 Massachusetts AvenueActive E17-412, Cambridge, M Department of Mat nematics logues. Taken together, these observations exemplify how assemmicrotubul (Dated: October 23, 2013) BASICS 77biomimetic Massachusetts Av blages of animate microscopic objects exhibit collective between mi Active nematics PACS numbers:1 a Jörn Dunkel1, ⇤ b d In 2d, the symmetric order-parameter tensor Q(t Department of Mathematics, Massachusetts In + + PACS numbers: with 77 Massachusetts Avenue E17-412, Cambridge, PEG Depletion force BASICS Qij = Qji , (Dated: October 23,can 2013) This be Tr Q = 0, BASICS numbers: In 2d, thePACS symmetric can be order-parameter represented as tensor Q(t, x, y) Microtubules with ✓ ◆ In 2d, the symmetric order-parameter tens Motor Time + µ Kinesin clusters force with Q = . where the u Q = Q , Tr Q = 0, (1) BASICS This can b ij ji c Dogicµ lab (Brandeis) Nature 2012 S= > 0,0, we Q = Q , Tr Q ij ji can be represented as whereas n = Defining In 2d, the order-parameter tensor Q(t, x, y) ✓ Q-tensor ◆ nosymmetric head or tail order-parameter canµbe represented as p th Q= . 2+µ 2 ,(2) ◆ ✓ = µ µ Q= Qij = Qji , Tr Q = 0, (1) . where the Defining the eigenvalues of Q are given by µ S > 0, we n be represented as pDefining We start whereas n ± 2 2 =✓ +◆ µ , (3) ⇤ =± energy dens p µ (rQ) (rrQ) 2 = (@k Qij )(@k Qij ) (11b) = (@k @n Qij )(@k @n Qij ). (11c) Matrix field theory Note that the potential cannot contain odd-power terms 2k+1 since Tr Q = 0 in 2D. Consider the corresponding field equation F @ Q + v @ Q = (12) t ij k k ij To obtain closed equation, we mustQ express v = (vk ) in ij terms of Q. We discuss two possible choices where v is the advection velocity and vk = D @n Qnk (16a 2 4 F a @ Tr Q b @ Tr Q vk + = D @n (Qnj Qjk ) (16b = Qij 2 @Qij 4 @Qij 2 a response coefficient2 with unit where the constant D is @(rQ) @(rrQ) 2 4 @ + @ @ . (13) 2 k k n length /time. the crucial di↵erence between th 2 Note @(@k Q ) 2 @(@ @ Q ) ij k n ij two closure conditions: Eq. (16a) assumes that active LC biological networks ! dunkel@math.mit.edu Tokyo rail network by Physarum plasmodium Tero et al (2010) Science dunkel@math.mit.edu