Sneak Preview: The Revised PHS FCOI Regulations Grace Park, COI Administrator
advertisement

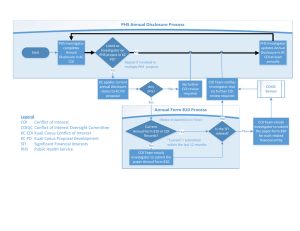
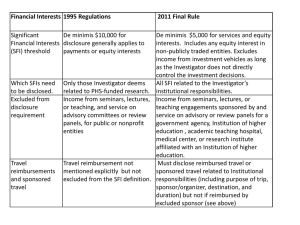
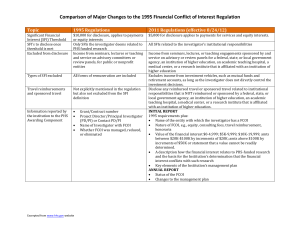
Sneak Preview: The Revised PHS FCOI Regulations Grace Park, COI Administrator Office of Research Agenda • • • • • Background Highlights Implementation Plan Contact Information Questions Timeline • In 1995, the Public Health Service (PHS)* published regulations to promote objectivity in research • The final rule revising the 1995 PHS regulations were issued on August 25, 2011 • Implementation of the revised regulations by August 24, 2012 Revisions Definition of Significant Financial Interest (SFI) - Threshold 1995 Regulations • Must disclose if aggregate payments or equity interests are greater than $10,000 2011 Regulations • Must disclose if aggregate payments or equity interests are greater than $5,000 – Privately held equity interest at zero – Travel reimbursements at any amount What Must Be Disclosed 1995 Regulations • Only Significant Financial Interests (SFI) the Investigator deems related to PHS-funded research 2011 Regulations • All SFIs related to Investigator’s institutional responsibilities Definition of Institutional Responsibilities • Investigator’s professional responsibilities on behalf of the Institution – Research – Research consultation – Teaching – Professional practice – Institutional committee memberships Determination of Relatedness 1995 Regulations • Investigator bore the responsibility for determining the relatedness of a SFI to the PHS funded research as part of the disclosure process 2011 Regulations • The Institution has the responsibility for determining whether an Investigator’s SFI is related to the PHS funded research Exclusions 1995 Regulations • Income from seminars, lectures, or teaching, and service on advisory committees or review panels, for public or nonprofit entities 2011 Regulations • Income from seminars, lectures, or teaching engagements sponsored by and service on advisory or review panels for a federal, state, or local government agency, an institution of higher education as defined at 20 U.S.C. 1001(a), an academic teaching hospital, a medical center, or a research institute that is affiliated with an institution of higher education New Requirements FCOI Training 2011 Regulations • Investigator must complete FCOI training prior to engaging in PHS-funded research and at least every 4 years Public Accessibility 2011 Regulations • The Institution shall ensure public accessibility of information regarding FCOI of key personnel Implementation Plan • Inform and educate researchers and research administrators about changes • Develop and update website with new policies • Develop and publicize online training • Publicize when UCI will begin following new regulations Contact Information Grace Park, COI Administrator • parkgj@uci.edu (949)824-7218 Nadia Wong, COI Analyst • nadiaw@uci.edu (949)824-0012 Resources NIH Summary of Major Changes •http://grants.nih.gov/grants/policy/coi/summary_of_major_changes.do NIH Website •http://grants.nih.gov/grants/policy/coi/ UCI Conflict of Interest Website •http://www.research.uci.edu/ora/coi/index.htm Questions?