Differen ntial Resting-S
advertisement
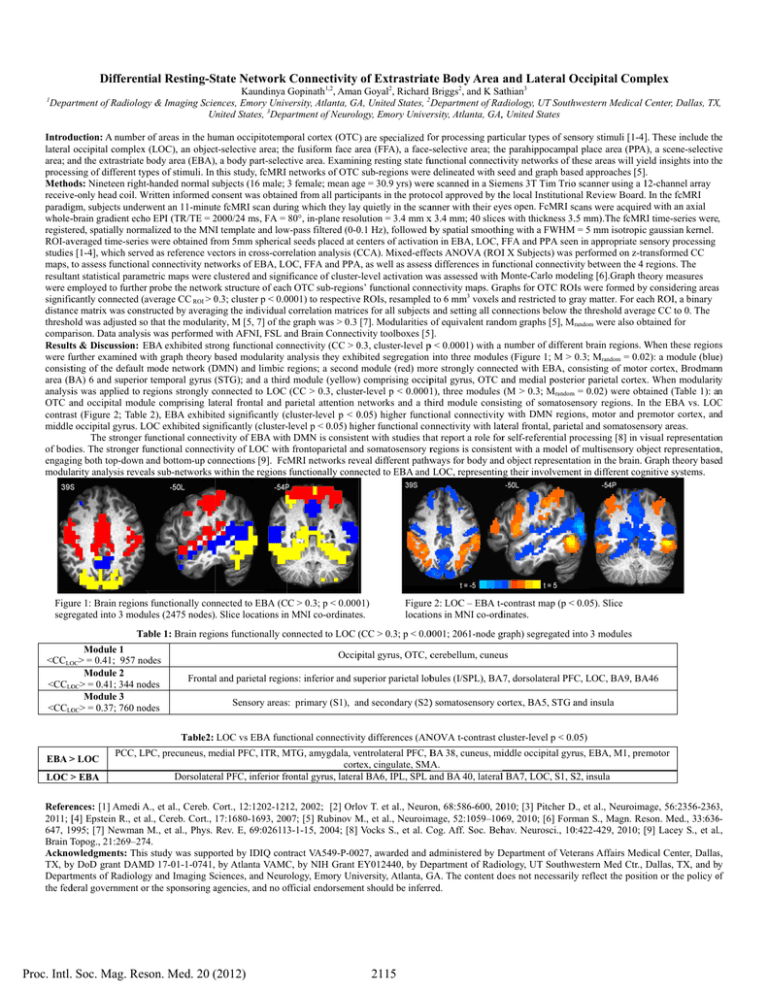
Differen ntial Resting-S State Network Connectivity of Extrastriatte Body Area and Lateral O Occipital Com mplex 1 Kaundinya Gopinath1,2, Aman n Goyal2, Richard Briggs2, and K Saathian3 Deparrtment of Radiolog gy & Imaging Scieences, Emory Univversity, Atlanta, GA A, United States, 2D Department of Raadiology, UT Southhwestern Medical C Center, Dallas, TX X, United U States, 3Department of Neurolo ogy, Emory Univer ersity, Atlanta, GA,, United States Introd duction: A numberr of areas in the hu uman occipitotemp poral cortex (OTC) are specialized ffor processing partticular types of sennsory stimuli [1-4]]. These include th he lateral occipital complex x (LOC), an object-selective area; th he fusiform face area a (FFA), a face--selective area; thee parahippocampaal place area (PPA A), a scene-selectiv ve ning resting state fuunctional connectiivity networks of tthese areas will yieeld insights into th he area; annd the extrastriate body area (EBA), a body part-selecctive area. Examin processing of different ty ypes of stimuli. In this study, fcMRI networks of OTC sub-regions weree delineated with seeed and graph based approaches [5]. ge = 30.9 yrs) weree scanned in a Siem mens 3T Tim Trioo scanner using a 112-channel array Methoods: Nineteen right-handed normal subjects (16 male; 3 female; mean ag receivee-only head coil. Written W informed consent c was obtain ned from all particiipants in the proto col approved by thhe local Institutionnal Review Board. In the fcMRI paradiggm, subjects underrwent an 11-minutte fcMRI scan duriing which they lay y quietly in the scaanner with their eyyes open. FcMRI scans were acquireed with an axial whole--brain gradient ech ho EPI (TR/TE = 2000/24 2 ms, FA = 80°, in-plane resolution = 3.4 mm x 3.4 mm; 40 slicess with thickness 3..5 mm).The fcMR RI time-series were, registeered, spatially norm malized to the MNI template and low w-pass filtered (0-0 0.1 Hz), followed bby spatial smoothiing with a FWHM M = 5 mm isotropicc gaussian kernel. c of activatioon in EBA, LOC, F FFA and PPA seenn in appropriate seensory processing ROI-avveraged time-seriees were obtained frrom 5mm sphericaal seeds placed at centers studiess [1-4], which serv ved as reference veectors in cross-corrrelation analysis (C CCA). Mixed-effeects ANOVA (ROII X Subjects) was performed on z-trransformed CC maps, tto assess functionaal connectivity nettworks of EBA, LO OC, FFA and PPA A, as well as assesss differences in funnctional connectivvity between the 4 regions. The resultaant statistical param metric maps were clustered c and signiificance of cluster--level activation w was assessed with M Monte-Carlo modeeling [6].Graph theory measures were employed to further probe the networrk structure of each h OTC sub-region ns’ functional connnectivity maps. Grraphs for OTC ROIs were formed byy considering areass ( CC ROI > 0.3; 0 cluster p < 0.0 0001) to respectivee ROIs, resampledd to 6 mm3 voxels aand restricted to grray matter. For eacch ROI, a binary significcantly connected (average distancce matrix was consstructed by averaging the individual correlation matricces for all subjects and setting all connnections below thhe threshold averaage CC to 0. The threshoold was adjusted so that the modularrity, M [5, 7] of thee graph was > 0.3 [7]. Modularities of equivalent randdom graphs [5], Mrandom were also obbtained for compaarison. Data analyssis was performed with AFNI, FSL and a Brain Connecttivity toolboxes [5 ]. Resultts & Discussion: EBA E exhibited strong functional con nnectivity (CC > 0.3, 0 cluster-level p < 0.0001) with a number of differeent brain regions. W When these region ns were fu further examined with w graph theory based b modularity analysis they exhiibited segregation into three modulees (Figure 1; M > 0.3; Mrandom = 0.022): a module (blue e) consistting of the defaultt mode network (D DMN) and limbic regions; a second module (red) morre strongly conneccted with EBA, coonsisting of motorr cortex, Brodmann area (B BA) 6 and superio or temporal gyrus (STG); ( and a third d module (yellow)) comprising occippital gyrus, OTC aand medial posterrior parietal cortexx. When modularitty analysiis was applied to regions r strongly connected c to LOC (CC > 0.3, clusteer-level p < 0.00011), three modules (M > 0.3; Mrandomm = 0.02) were obttained (Table 1): an a OTC aand occipital modu ule comprising lateral frontal and parietal p attention networks n and a thhird module consiisting of somatoseensory regions. In the EBA vs. LOC C contrasst (Figure 2; Tablee 2), EBA exhibitted significantly (ccluster-level p < 0.05) 0 higher functtional connectivityy with DMN regioons, motor and prremotor cortex, an nd middlee occipital gyrus. LOC L exhibited sign nificantly (cluster--level p < 0.05) hig gher functional connnectivity with latteral frontal, pariettal and somatosensory areas. The stronger functional connecctivity of EBA with h DMN is consisteent with studies thhat report a role forr self-referential processing [8] in viisual representation of bodies. The stronger functional f connecttivity of LOC with h frontoparietal an nd somatosensory rregions is consisteent with a model oof multisensory obbject representation n, onnections [9]. FcMRI networks rev veal different pathw ways for body andd object representaation in the brain. G Graph theory based engagiing both top-down and bottom-up co modulaarity analysis reveeals sub-networks within w the regions functionally conn nected to EBA and LOC, representinng their involvemennt in different cognnitive systems. Figuure 1: Brain region ns functionally con nnected to EBA (C CC > 0.3; p < 0.000 01) segrregated into 3 mod dules (2475 nodes). Slice locations in n MNI co-ordinatees. Figuree 2: LOC – EBA t--contrast map (p < 0.05). Slice locatioons in MNI co-orddinates. ons functionally co onnected to LOC (CC ( > 0.3; p < 0.00001; 2061-node ggraph) segregated iinto 3 modules Table 1: Brain regio Module 1 <CCLOOC> = 0.41; 957 no odes Module 2 <CCLOOC> = 0.41; 344 no odes Module 3 <CCLOOC> = 0.37; 760 no odes Occip pital gyrus, OTC, ccerebellum, cuneuus ons: inferior and su uperior parietal lobbules (I/SPL), BA A7, dorsolateral PFC, LOC, BA9, BA A46 Frontall and parietal regio Sensory areass: primary (S1), and a secondary (S2)) somatosensory coortex, BA5, STG aand insula Table2: LOC vs EBA funcctional connectivitty differences (AN NOVA t-contrast ccluster-level p < 0.05) EBA > LOC LOC > EBA PCC, LPC, L precuneus, medial m PFC, ITR, MTG, M amygdala, veentrolateral PFC, B BA 38, cuneus, miiddle occipital gyrrus, EBA, M1, premotor corttex, cingulate, SMA A. Dorsolaterral PFC, inferior frrontal gyrus, lateraal BA6, IPL, SPL aand BA 40, laterall BA7, LOC, S1, S S2, insula A et al., Cereb. Cort., C 12:1202-121 12, 2002; [2] Orlo ov T. et al., Neuroon, 68:586-600, 20010; [3] Pitcher D D., et al., Neuroimaage, 56:2356-2363 3, Refereences: [1] Amedi A., 2011; [[4] Epstein R., et al., Cereb. Cort., 17:1680-1693, 200 07; [5] Rubinov M., M et al., Neuroim mage, 52:1059–10669, 2010; [6] Form man S., Magn. Reson. Med., 33:636 6647, 19995; [7] Newman n M., et al., Phys. Rev. E, 69:026113-1-15, 2004; [8] Vocks S., et al. C Cog. Aff. Soc. Behhav. Neurosci., 100:422-429, 2010; [[9] Lacey S., et all., Brain T Topog., 21:269–27 74. Acknoowledgments: Thiis study was suppo orted by IDIQ con ntract VA549-P-00 027, awarded and aadministered by D Department of Veteerans Affairs Meddical Center, Dallass, TX, byy DoD grant DAM MD 17-01-1-0741, by Atlanta VAMC C, by NIH Grant EY012440, E by Deepartment of Radioology, UT Southw western Med Ctr., D Dallas, TX, and by Departtments of Radiolog gy and Imaging Scciences, and Neurology, Emory Uniiversity, Atlanta, G GA. The content d oes not necessarilyy reflect the positiion or the policy of o the fedderal government or o the sponsoring agencies, a and no official endorsemen nt should be inferrred. Proc. Intl. Soc. Mag. Reson. Med. 20 (2012) 2115





