How we use animal studies to understand recovery from brain injury
advertisement

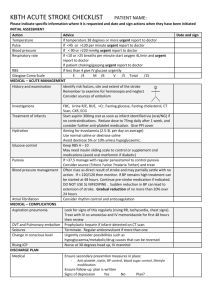
How we use animal studies to understand recovery from brain injury Ann M. Stowe, PhD Assistant Professor Neurology & Neurotherapeutics Overview Introduction to clinical stroke Post-stroke plasticity in non-human primates Stroke models in rodents Methods to promote recovery after stroke Educational resources for the use of animals in biomedical research General considerations The brain is highly aerobic tissue Dependent upon a steady supply of well-oxygenated blood 650 – 750 ml of arterial blood /minute 15% of total cardiac output 20% of body’s total O2 consumption Global interruption in blood flow results in loss of consciousness within 10 seconds! Irreversible CNS injury occurs of blood flow drops to less than 15 ml / 100 gm tissue / minute Blood flow to the brain is maintained at a constant rate over a wide range of blood pressure (autoregulation) Stroke Global mortality, all causes: 65 million people Global mortality, stroke: 6.5 million people – 10% In the US, stroke #1 cause of long-term adult disability 800,000 U.S. strokes/yr $73 billion annually (US) to provide long-term care Stroke Center, University Hospital Coronal view Ischemic stroke WebMD 88% of all strokes are ischemic Thrombotic – blood clots formed in the artery (50%) Embolic – blood clots dislodged from the body and trapped in arteries in brain Stroke • Infarct • Intact cortex Tissue necrosis, neuronal death, possible loss of pre-lesion function • Peri-infarct • CBF is 20-50% of normal values • Cells are at risk of apoptosis or necrosis Infarct Peri-infarct Middle cerebral territory Lateral view Primary and secondary motor cortices in primates Primary motor cortex Premotor cortex Preuss et al., 1996; Dum and Strick, 2002; Dancause et al., 2005; owl monkey Neurons that are interconnected to the infarct will undergo molecular responses immediately following infarct induction medial rostral Primary motor cortex hand representation To sensory hand Premotor cortex hand digit wrist face proximal no response Previous work in the squirrel monkey model has highlighted neuronal changes following an infarct medial rostral Infarct An infarct was induced in 30% of the M1 hand representation (Nudo and Milliken, 1996) medial rostral Infarct Following spontaneous recovery, there is a further loss of hand representation (Nudo and Milliken, 1996) medial rostral Infarct Following rehabilitative motor skill training, there was an actual increase in M1 hand representation (Nudo et al., 1996) medial rostral Infarct Dancause et al, J Neurosci, 2005 To sensory hand PMv hand neurons undergo axonal sprouting to novel targets in primary somatosensory cortex (Dancause et al., 2005) Findings: Disuse of the hand can decrease the number of neurons that directly control hand movement after brain injury Rehabilitation after stroke directly affects neuronal plasticity during recovery Recovery after brain injury takes months to complete in larger brains, especially during new connections Stroke models in rodents – why? Much more readily available Behavioral recovery can be monitored in rats and mice We know their genetics… …which allows for genetic manipulation Develop various models to ask different questions Myriad ways we can quantify injury and repair – genetic, molecular, behavioral, imaging Variety of rodent models of stroke: University of Glasgow Glasgow Experimental MRI Centre Luo et al., JCBFM (2008) 28, 973–983 Middle Cerebral Artery Occlusion (tMCAo) Procedure Intraluminal filament is threaded to the origin of the MCA, with retraction comes brain reperfusion. Infarct Volume 2,3,5-triphenyltetrazolium chloride (TTC) Transient: Excellent for looking at post-stroke inflammation Permanent: Much larger infarct volumes Imaging techniques to measure infarct volumes T2 weighted MRI 24h after permanent MCAo to measure edema University of Glasgow Glasgow Experimental MRI Centre Diffusion tensor imaging after permanent MCAo to measure white matter tracks Water moves along the axons of neurons faster than in cerebral cortex. This allows for quantification of direction based on the rate of diffusion. University of Glasgow Glasgow Experimental MRI Centre This means we can track the in vivo progression of the infarct, along with behavioral recovery, to assess the efficacy of drug or behavioral interventions 60-min tMCAo PBS (n=14), WT B cell (n=12), RHP B cell (n=11) Unpublished data Motor recovery can be measured as a secondary outcome Human CD20 transgenic mice B cells depleted with Rituximab 8-12 week males 60-min tMCAo (n=11 WT, n=13 B cell-depleted) Unpublished data Blood-Brain Barrier - Endothelial Cells • High-resistance tight junctions • Capillaries are 40m apart • No transcellular pathways Pardridge, 1997 Blood-Brain Barrier - Pericytes • Share basement membrane with EC • Antigen-presenting properties • May regulate blood vessel growth and EC proliferation in quiescent cortex Pardridge, 1997 Blood-Brain Barrier - Astrocytes •Foot processes cover more than 99% of brain capillary surface •Site of p-glycoprotein, product of the multidrug resistance gene •Effective efflux system Pardridge, 1997 The BBB is physically uncoupled in areas of ischemic injury • Angiogenic factors facilitate endothelial/pericyte dissociation and disruption of tight junctions • Astrocyte end-feet withdraw from the vasculature • Increase in vascular permeability into peri-infarct tissue • Cerebral edema Post-Ischemic Inflammation: Leukocyte diapedesis occurs in the postcapillary venules modified from Eltzschig and Collard, 2004 • Selectins mediate rolling along the vessel wall • Integrins mediate firm adherence to the vessel wall Flow cytometry can be used to quantify leukocyte populations within the injured (i.e. ischemic) hemisphere, spleen, or blood Abcam.com B cells support post-stroke neurogenesis Ipsilateral WT B cell-depleted WT Contralateral B cell-depleted Scale bar = 20µm hCD20Tg mice, WT littermate controls All receive Rituximab Bottom border- subgranular zone Dendrites extending into the molecular layer Unpublished data Recap: Several models for inducing stroke, can ask different questions Concurrent quantification of outcomes Use of genetic manipulation to generate new mouse strains Look at important mechanisms that can not be studied in the clinical population Help to understand the contribution of genetic, environmental, and physiological factors to stroke outcome in the individual “Preconditioning” The presentation of a non-injurious stimulus that promotes adaptive responses at the level of the cell, tissue, organ, and/or whole animal to afford protection against an injurious or lethal intervention. “Tolerance” The state of relative resistance to a normally injurious or lethal intervention. (Dirnagl et al., Trends Neurosci., 2003) In Vivo Preconditioning Stimuli Local • brief ischemia • mild trauma Systemic • hypoxia and hypoxia-mimetic drugs • hyperoxia, hypoglycemia, caloric restriction • heat shock • cytokines, LPS, anesthetics, metabolic inhibitors, antibiotics • distant tissue ischemia (“remote” PC) • exercise Tissue or Cellular Response necrosis apoptosis tolerance none * * Magnitude of Stress Sustained exercise – but not the magnitude of exercise – creates a unique B cell phenotype in the blood Unpublished data Hypothesis: Exercise-mediated changes in adaptive immunity are lost with detraining stroke SEDENTARY (SED) 3 or 5 week sedentary period 3 days Flow cytometry on brain and spleen stroke 3 week exercise period EXERCISE (EX) 3 days Flow cytometry on brain and spleen stroke DETRAINING (DET) 3 week exercise period 2 week sedentary period 3 days Flow cytometry on brain and spleen Detrained animals exhibit increased infarct volumes SED EX DET Unpublished data Exercise intensity induces a non-linear, dose-dependent increase of immune cells in the ischemic brain that is lost after detraining All leukocytes in the brain All leukocytes 40000 30000 20000 10000 2000000 1000000 0 * Ex26 Ex28 Ex29 Ex30 Ex31 Ex32 Ex33 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 Ex27 Ex34 ** *** ** 30000 * * *** 20000 10000 0 Det17 Det18 Det19 W 1 W 2 W 3 Det16 W 1 W 2 W 3 Det15 W 1 W 2 W 3 W 1 W 2 W 3 Det14 W 1 W 2 W 3 W 1 W 2 W 3 Det13 W 1 W 2 W 3 W 1 W 2 W 3 W 1 W 2 W 3 Det12 30000 40000 50000 Det20 R2 = 0.02860 1500000 1000000 500000 0 Det11 20000 2000000 *** ** 40000 10000 All leukocytes DETRAINING * 0 Average Wheel Rotations # cells/hemisphere (mean/SD) 60000 W 1 W 2 W 3 0 W 1 W 2 W 3 DETRAINING * ** Ex25 Average number of rotations/week ** * *** # cells/hemisphere (mean/SD) *** 50000 50000 R2 = 0.8423 3000000 60000 W 1 W 2 W 3 EXERCISE Average number of rotations/week EXERCISE 0 10000 20000 30000 40000 Average Wheel Rotations 50000 Unpublished data Recap: Spontaneously hypertensive rodents Obese and aged rodents Other environmental factors- exercise vs. sedentary lifestyle We can use these interventions to determine the mechanisms by which lifestyle and genetics can contribute to injury and recovery after stroke. http://www.pewinternet.org/2015/07/01/c hapter-7-opinion-about-the-use-ofanimals-in-research/ Bringing up the concept of animals in biomedical research Pew research into the public opinion of animal research American Physiological Society Advocacy and Outreach Presentations available online http://www.the-aps.org/mm/SciencePolicy/Advocacy/Research-Benefits NIH RePORT website- search by any disease http://report.nih.gov/NIHfactsheets/Default.aspx?key=S#S Questions? AND THANKS !