Document 10579426
advertisement

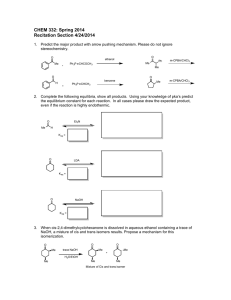
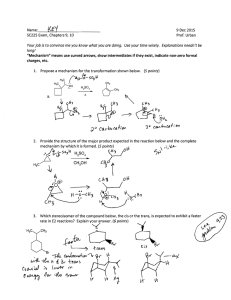
CHEM 332. Spring 2014 Problem Set 4 Prof Donald Watson 1. The Witting reaction is useful for placing double bonds in less stable positions. For example, the following transformation is easily accomplished using a Witting reaction. O CH 2 (a) Show how you would use a Witting reaction to do this. (b) Show how you might do this without using a Wittig reaction, and explain why the Wittig reaction is a much better synthesis. 2. Predict the product for the following Wittig reactions with arrow-pushing mechanism. Please do not ignore stereochemistry. O benzene H Ph 3P CHCH3 H Ph 3P CHCO 2Et O ethanol O benzene Ph 3P CHCH 2CH 2CH3 O ethanol Me 3. Ph 3P CHCN The Baeyer-Villiger oxidations of the substituted diphenyl ketones proceeds as indicated. Please rationalize this result using mechanism. O OMe OMe O m-CPBA O CHCl3 NO 2 O O m-CPBA NO 2 CHCl3 O 4. Predict the product for the following Baeyer-Villiger Oxidation with arrow-pushing mechanism. O O Me O Me Me Me Me Me CF3COOOH CF3COOOH Me CF3COOOH O H CF3COOOH 5. Complete the following equilibria, show all products. Using your knowledge of pka’s predict the equilibrium constant for each reaction. In all cases please draw the expected product, even if the reaction is highly endothermic. O HCl O- Me Na + K eq = LDA EtOH K eq = NaOH BuOH K eq = EtONa Ph H K eq = O Et 3N K eq = 6. Propose a mechanism for the following reaction: O O O HO EtOH O 7. Starting from cyclohexanone, How would you synthesis the following compounds using Stork enamine synthesis. O O O O OEt Me O O CN Ph O 8. Predict the product with arrow-pushing mechanism. 1. LDA, -78 °C THF O Me 2. CH3CH 2Br O NaOH/H 2O Δ O O O Me O Excess I 2, NaOH 1. (CH 2=CH) 2CuLi, THF 2. CH 2=CHCOCH3 O LDA, -78 °C THF Me Br 1. LDA, -78 °C THF O 2. BrCH 2CO 2CH3 O Excess Br 2, NaOH H 2 NaOH/H 2O O 9. The following sequence of reaction is called as Robinson Annulation. It is one of the methods for building substituted cyclohexenones and this synthetic strategy was used in several complex natural product syntheses. Identify products A and B. O Me Me O NaOEt/EtOH, -10 oC A NaOH/H 2O Δ B 10. Show how you might carryout the following synthetic transformation: O O Me Me 11. Please draw a arrow-pushing mechanism for the following reactions. Me N O Me Δ Me OMe Me Δ NH 3 OEt MeOH O HCl/H 2O OMe OH O HCl/EtOH O Me O NaOH/H 2O Me OH MeOH