Microemulsion Polymerization Eric W. Kaler Jen O’Donnell Kevin Hermanson
advertisement

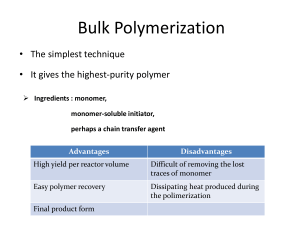
Microemulsion Polymerization M M M M P• M M PM• M M IM• M Eric W. Kaler M I• M M• M M Department of Chemical Engineering M University of Delaware M Newark, DE 19716 M M M M M Jen O’Donnell Kevin Hermanson CarlosMCo (U. Cincinnati) Renko de Vries (Wageningen U.) M Characteristics and Structures Emulsion: •Two Phase •Energy Needed to Form •Opaque •Monomer Drops > 1 µm Microemulsion: •One Phase •Spontaneous •Transparent/ •Swollen Micelles ( D < 20 nm) Why Study Microemulsion Polymerization? Produces nanosized (~15 nm) latex particles smaller than those obtainable by emulsion polymerization Polymerization in a confined environment may lead to unique polymer morphologies, e.g. tacticity and knotting. “Paint” the walls of a microporous material “Knotted” polymer chain in solution Dry “Seeds” for emulsion polymerization Extremely high MW ( ~20 million daltons) are readily made Outline • • • • • • • Problems and Model Mixtures How to Make Microemulsions Microstructures Polymerization – Kinetics and Model Structure Evolution Multiple Additions The End! Ternary Phase Diagrams at 60ºC Added degree of freedom from mixing surfactants is used to tune one phase oil-in-water microemulsions Gibbs Phase Prism at 60ºC Mixing ratio sets surfactant chemical potential Free Radical Thermal Polymerization Monomers n-C6MA n-C4MA t-C4MA Styrene Water Solubility 60°C (mM) ~ 0.4 3.4 4.3 4.6 Tg (°C) -5 20 128 106 kp 60°C (L mol-1 s-1) 995 1015 1140 340 Reaction at 60°C H2C CH3 O O CH2 H2C CH2 H2C CH2 H3C H2C CH3 O O CH2 H2C CH2 H3C H2C CH3 O O H3C C CH3 CH3 HC CH2 A Basic Recipe • Surfactant mixture to tune phase behavior Cationic surfactants DTAB DDAB • Monomer with low water solubility - hexylmethacrylate (0.4 mM) • Polymer with low Tg - polyhexylmethacrylate (-5°C) • Radical initiator with simple dissociation kinetics - V50 (not persulfates) A Simple View Of Microemulsion Polymerization M Initiation by IM• M M M M Chain Transfer M P• M M• M IM• M PM• M Propagation M M M M I• M M M M Initiation by M• M M M M # Micelle ~ 103 # Particles 5 nm V – 50 Polymerizations 1. Rapid polymerization 2. 100% conversions 3. Rate profile parabolic 4. Average maximum rate at about 39% conversion Microemulsion Polymerization – John Morgan Modeling the V-50 Rate Curves • Fundamental rate equation for addition polymerization: c = monomer concentration at polymerization locus [ R • ] = concentration of propagating radicals ∂c − = k p [ R • ]c ∂t • Microemulsion conversion form: ∂f = ∂t k p N * (t )c( f ) Mo Mo = monomer concentration at polymerization locus (M) N* = propagating radical concentration in whole microemulsion (M) Modeling the V-50 Rate Curves (cont.) • Assumption: Monomer concentration within polymer particles given by: c = co (1 – f ) co = initial concentration of monomer, M • Entry rate is constant; all radicals remain active N*(t) = ρot ρo = rate of radical entry, M s-1 Modeling the V-50 Rate Curves (cont.) k p N * (t ) c ( f ) k p c o ρ o ∂f = = t (1 − f ) • Rate Equation: ∂t Mo Mo Or Parameter: Conversion: ∂f = At (1 − f ) ∂t k p co ρ o A= Mo 1 2 f = 1 − exp(− At ) 2 Modeling Implications Rate Maximum : f′= A e Conversion at Maximum Rate : Time of Maximum Rate : f = 1 − e −0.5 = 0.39 t = A−1/ 2 • Dependence on Initiator Concentration: Assume ρo = 2kd [I] then A goes as [I] Measured Rate Constants – Propagation Rate Constant • kp = 995 M-1s-1 (Pulsed Laser Polymerization) – Initiator Decomposition Rate Constant • kd = 3 x 10-5 M-1s-1 (Literature) – Initial Monomer Concentration in Droplet • C0 = 1.0 M (SANS) – Initial Monomer Concentration in microemulsion • M0 = 0.257 (Formulation) N.B. No fitted parameters! Comparison with Experiment Quantitative Agreement Through to Full Conversion Kinetic Modeling: Monomer Concentration at Locus of Polymerization Carlos Co, Renko de Vries How Does the Microstructure Evolve? Case II Case III Flory-Huggins for both latex particles and micelles. All monomer is taken up by latex particles at ~ 5% conversion. Flory-Huggins for latex particles and curvature energy for micelles. Monomer partitions between latex and micelles. No swelling of polymer latex. Polymerization occurs in shell of latex particles with monomer concentration equal to that in the micelles. Monomer Concentration Case I Conversion Conversion Conversion Monomer concentration is approximately linear with conversion. Can differentiate only using Small Angle Neutron Scattering (SANS). Small Angle Neutron Scattering (SANS) 2D Detector Sample Neutron Source q= λ 4π ⎡ θ ⎤ sin ⎢ ⎥ λ ⎣2⎦ θ q~ I(q) = n P(q) S(q) P(q) ⇒ Form Single particle properties ⇒ Factor (size, shape, composition) Structure ⇒ S(q) ⇒ Factor Relative positions of particles due to interactions 1 Length q What Can SANS Tell Us? Particle Scattering Observed Scattering I(q) Micelle Scattering q q q How does the SANS spectra change as the microstructure evolves from micelles to a mixture of polymer particles and micelles? Reactor for Online Scattering During Polymerization Online SANS / Kinetics Experiments C6MA (DTAB/DDAB) -1 Intensity (cm ) 1000 100 Increasing Particle Size and Number Density Decreasing Micelle Size 0% 10% 38% 65% 85% 96% 100% 10 0.10 0.01 1 -1 q (Å ) Gradual shifts in SANS spectra indicate that monomer partitions between the micelles and the polymer particles. Case I is incorrect. Connection of particle size to MWD • Basic Idea – Growing chain of L segments was initiated at an earlier time t1 – At t1, calculate Δt for one propagation event – Number of chains initiated during Δt is N(L) – Assume single chain particles • Final result is analytical • See Morgan and Kaler, Macromolecules, 1998 Predicted MW and Size Distributions SANS Model for Online SANS Spectra Effective HS Interactions Form Factors Polymer Discretize Model-Predicted Particle Size Distribution Micelle Calculate model intensities using Vrij’s analytical equation Three Adjustable Parameters REHS Rmin Rmaj Online SANS Modeling Results Micelle Dimensions C6MA (DTAB/DDAB) (Fitted Parameters) 0% 100 0% Minor Radius (Å) 29 23 20 Aspect Ratio 2.1 2.3 2.2 Minor/HS Radius Ratio 1.6 1.7 1.6 65% 100% 10 Particle Size Distribution (Model Predictions) 0.20 0.10 0.08 0.06 0.04 1 0.02 0% 0.01 -1 Intensity (cm ) 1000 65% 100% 65% 100% Avg Radius (Å) 160 130 Stdev (Å) 50 70 -1 q (Å ) To within the accuracy of the predicted particle size distributions, the polymer particles are not swollen by monomer. SANS Model Fitting Results Particle size distribution model is consistent with SANS Micelle size decreases steadily with increasing conversion Validation of SANS Swelling Experiments (n-C4MA) -1 Intensity (cm ) 1000 100 4% Online Swelling 33% Online Swelling 75% Online Swelling 10 1 0.00 0.05 0.10 0.15 -1 q (Å ) cryo-TEM by Stefan Burauer (Universitaet zu Koeln) Molecular Weight Distribution Conv. (%) Mw (106) Mw/Mn 4 18.6 1.9 37 26.1 1.2 100 22.4 1.4 By Pat Cotts, Dupont Molecular Weight Distributions n-C6MA n-C4MA dw/d(log M) 95% 94% 76% 79% 60% 43% 33% Molecular Weight (106) GPC/MALLS/RI by Patricia Cotts (DuPont) Polystyrene Exceeds Chain Transfer Limit Styrene kp dw/d(log M) 91% PM • kp/ktr ratio sets characteristic P•+M ktr P+M• limiting molecular weight 70% 53% 27% Molecular Weight (106) Styrene free-radical polymerizations (60°C) limited to ~2·106 daltons. MW~15 ·106 polystyrene is consistently prepared by microemulsion polymerization Summary: A Simple Model for Microemulsion Polymerization Rate Equation ∂f = At (1 − f ) ∂t ⎛ t2 ⎞ Conversion : f = 1 − exp⎜⎜1 − A ⎟⎟ 2⎠ ⎝ k p c oρ o ⎛ 1⎞ Conversion at max rate : f = 1 − exp⎜ − ⎟ = 0.39 ⎝ 2⎠ Mo is known V50 Concentration Predictions for Particle Size and Molecular Weight Distributions dw/d(log M) A= Kinetic Predictions Molecular Weight (106) Initial Step 1 1 2 How to Increase Polymer Loading? M M M Step 2 Refill 3 …Sequential Addition Polymerization 4 M M Kinetics •Radicals present from prior monomer additions N* = ρο t + N*o Additional Steps 5 ∂f k p co (1 − f ) = 2 k d [I ] t + N * o ∂t Mo ⎛ k pco f = 1 − exp ⎜⎜ − k d [I ] t 2 + N *o t ⎝ Mo ( ( ) ⎞ ⎟⎟ ⎠ ) Determine Cmon from Single Addition Kinetics C6MA/DTAB/DDAB (5% Total Surfactant) Single Addition Kinetics Monomer Partitioning Map Single Addition Polymerization (Scaled*) wt%mon=1.90 1.4 wt%mon=1.43 0.008 0.002 0.8 90 wt%mon=0.475 0.004 1.0 . =1 wt%mon=0.95 0.006 1.2 on m Monomer Concentration Cmon(M) 0.010 % wt d(Conversion)/d(time) (s-1) 0.012 0.6 0.4 Increase Total Monomer wt% mon =0 0.2 Concentration5 .47 0.0 0.000 0.0 0.2 0.4 0.6 0.8 1.0 Conversion (f) *0.95% scaled 1.5X, 1.43% scaled 2X, 1.90% scaled 2.5X 0.0 0.2 0.4 0.6 Conversion 0.8 1.0 Determine Cmon for Multiple Addition Polymerization Cmon=Co(1-f) Monomer Partitioning Map Multiple Addition Polymerization Monomer Partitioning Map Single Addition Polymerization 0.475% Monomer 0.475% Polymer 1.4 0.475% Monomer 0.95% Polymer 1.2 1.0 0.475% Monomer 1.43% Polymer 0.8 0.6 0.4 0.2 New Co 0.0 0.0 0.2 0.4 0.6 Conversion (f) 0.8 1.0 Monomer Concentration Cmon(M) Monomer Concentration Cmon(M) 1.4 Addition 1 Co=0.66M Addition 2 Co=0.56M Addition 3 Co=0.47M Addition 4 Co=0.37M 1.2 1.0 0.8 Ad diti 1 on 0.6 0.4 0.2 Additio n 4 0.0 0.0 0.2 0.4 0.6 Conversion (f) 0.8 1.0 Multiple Addition Kinetics Multiple Addition Model ∂f k p c o (1 − f ) = 2 k d [I ] t + N *o ∂t Mo ( Measured vs. Predicted Kinetics Measured Kinetics 0.004 0.003 0.002 Addition 1 Addition 2 Addition 3 Addition 4 0.001 0.000 0.0 0.2 0.4 0.6 Conversion (f) 0.8 (offset*) Addition 1 0.008 Co decreases lowering reaction rate d(Conversion)/d(time) (s-1) d(Conversion)/d(time) (s-1) 0.005 ) 1.0 Addition 2 0.006 Addition 3 0.004 Addition 4 0.002 0.000 0.0 0.2 0.4 0.6 0.8 1.0 Conversion (f) The predicted data matched the measured data when N*o=0 * Addition 2 offset 0.001, Addition 3 offset 0.002, Addition 4 offset 0.003 Multiple Addition Particle Size Measurements Particle Size Distribution Contin Analysis Particle Size Predicted particle size if no new particles are formed 80 100 80 Experimentally Measured Particle Size 60 60 40 40 20 20 QLS Measured Particle Size Maximum Predicted Particle Size 0 0 0 10 4.6 20 30 Generation Number 8.8 12.6 % Polymer 40 16.2 50 19.4 *100) 100 25 0 3 5 3 Dia 4045 me 50 5 ter 5 60 65 1 12 22 32 42 0 max Diameter (nm) 120 Intensity (I/I 140 n itio d Ad After 43 additions latex contains 17% polymer and 4% surfactant Summary • Microemulsion polymerization produces small monodisperse particles • Initiator charge plays no role (with pH control…) • Reaction rate and MWD can be modeled with minimal assumptions and no free parameters • Microstructures “meter” monomer and control polymerization • Commercially interesting concentrations can be produced by sequential (or continuous) polymerization