MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering
advertisement

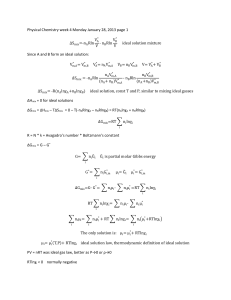
MSE 308 Thermodynamics of Materials Dept. of Materials Science & Engineering Spring 2005/Bill Knowlton Problem Set 12 1. For a regular solution, it is known for a two component system, that: ∆Gmix = ao X 1 X 2 + RT ( X 1 ln X 1 + X 2 ln X 2 ) (J/mole). Consider a system in which ao is -13,500 J/mole. xs . a. Derive ∆Gmix xs b. Derive ∆G1 . xs Derive ∆G 2 . Derive the activity coefficient for component 1. Derive the activity coefficient for component 2. Plot the activity coefficient for component 1 as a function of X2 over a temperature range from 300K to 1000K on one graph using a mathematical program that is not Excel. g. Plot the activity coefficient for component 2 as a function of X2 over a temperature range from 300K to 1000K on one graph using a mathematical program that is not Excel. h. Plot ∆Gmix as a function of X2 over a temperature range from 300K to 1000K on one graph using a mathematical program that is not Excel. c. d. e. f. 2. For an nonregular (subregular) solution, a two parameter solution model is given by: T ∆Gmix = ao X 1 X 2 (1 + ) + RT ( X 1 ln X 1 + X 2 ln X 2 ) (J/mole). c xs a. Determine ∆Gmix . xs xs xs xs xs and ∆Smix and show whether or not ∆Gmix = ∆H mix − T ∆Smix b. Determine ∆H mix matches your answer in part a. xs xs xs . Show whether c. Find ∆G1 and ∆G 2 and use your answers to determine ∆Gmix xs agrees with your answer in part a. or not your answer for ∆Gmix d. Determine a1 and a2 (activities) 3. In the paper handed out in class: J. H. Hildebrand, Solubility. XII. Regular Solutions, Journal of the American Chemical Society, Vo. 51 (1929) p. 66-88 Describe five aspects of the paper that you can relate to what you have learned in this course. This is an open ended question. 1 of 1