Lennard Jones Potential
advertisement

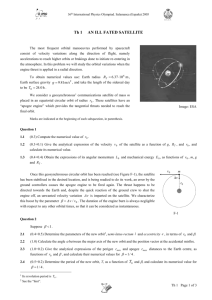
Lennard Jones Potential The anti-symmetry of parallel spin electrons makes neutral atoms resist being pushed together. The slight polarization of the electrons in one atom relative to another gives rise to a slight attraction between the atoms. The potential between two neutral atoms can be written as 1 12 6 v r 4 (1.1) r r Note that the potential minimum is at 11 5 v ' r 4 2 12 6 0 r r r 1 .89089872 r 2 rmin 1.1224620 1/6 vmin (1.2) 1 2 1 4 2 2 v '' rmin 4 r 7 13 12 6 r r 12 6 4 2 12 13 42 r r r 4 1 1 2 12 13 42 4 2 rmin 12 2 rmin (1.3) So that to a first approximation 1 M.P.Allen and D.J. Tildesley, Computer Simulation of Liquids, Oxford Science Publications, (1987,1989),p 9 1 2 v r v rmin v '' rmin r rmin 2 2 (1.4) r rmin 1 6 rmin On page 22 of Allen and Tildesley is the table Atom Source /k (0K) (nm) 2 H Murad and Gubbins 8.6 0.281 He Maitland, et al3 10.2 0.228 C Tildesley and Madden4 51.2 0.335 N Cheung and Powles5 37.3 0.331 O English and Venables6 61.6 0.295 F Singer et al7 52.8 0.283 Ne Maitland et al8 47.0 0.272 S Tildesley and Madden 183.0 0.352 Cl Singer et al 173.5 0.335 Ar Maitland et al 119.8 0.341 Br Singer et al 257.2 0.354 Kr Maitland et al 164.0 0.383 While the above is valuable for working with real systems, for today 1 1 6 (1.5) 12 r r The Boltzman constant k = 1.3807 10-23J/0K 1 eV = 1.6022 10-19 J 9 1 Rydberg = 0.5 Hartree = 13.6 eV aB = 5.29172 10-9 cm For He mHe = 4.0026 u me = 0.000549 u He 0.228 109 m 1aB / (5.29172 1011 m) 4.31 aB v r He 10.2 0 K 1.3807 1023 J / 0 K 1eV / 1.60219 10 19 J 0.5Hartree / 13.6eV 10.2 1.3807 0.5 102319 0.323 104 Hartree 1.60219 13.6 The Schroedinger equation for Helium atoms 1 and 2 is 12 6 2 R1 , R2 R1 R2 R1 R2 4 He R1 , R2 ( EHartree ) R1 , R2 He 2mHe / me He 2 S.Murad and K.E. Gubbins, "Molecular dynamics simulation of methane using a singularity free algorithm". In Computer modelling of matter, (ed. P. Lykos) ACS Symposium Series Vol. 86, pp. 62-712. American Chemical Society, Washington. 3 G.C. Maitland, et al, Intermolecular forces: their origin and determination, Clarendon Press, Oxford (1981) 4 D.J. Tildesley and P.A. Madden, Mol. Phys. 42, 1137-1156 (1981) 5 Mol. Phys. 30, 921-49 (1975) 6 Proc. R. Soc. Lond. A340, 57-80 (1974) 7 Mol. Phys. 33, 1757-95 (177) 8 G.C. Maitland,M.Rigby,E.B.Smith,W.A. Wakeham, Intermolecular forces: their origin and determination, Clanendon, Oxford (1981) 9 F. Bueche, Principles of Physics (third edition), McGraw Hill, 1965 Figure 1 h2m.dat is <H2> -1. LJfit was fitted to this by matching the curves at r min = 1.4 using (1.2) ..\wsteve\h2fit LennardJones.zip The H2 is not spin aligned so there is no statistical repulsion of the type assumed in the Lennard Jones. The curves are quite different.