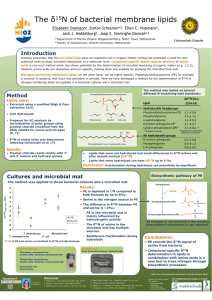
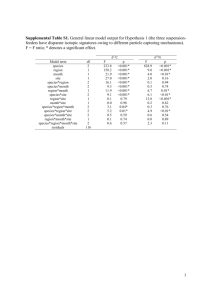
Nitrogen isotopes in bivalve shells from the Colorado River estuary:
advertisement

Nitrogen isotopes in bivalve shells from the Colorado River estuary: Evaluating a potential proxy for changes in riverine nutrient delivery by Robert D. Dietz A Prepublication Manuscript Submitted to the Faculty of the DEPARTMENT OF GEOSCIENCES In Partial Fulfillment of the Requirements for the Degree of MASTER OF SCIENCE In the Graduate College THE UNIVERSITY OF ARIZONA 2008 STATEMENT BY THE AUTHOR This manuscript, prepared for publication in Limnology and Oceanography, has been submitted in partial fulfillment of requirements for the Master of Science degree at The University of Arizona and is deposited in the Antevs Reading Room to be made available to borrowers, as are copies of regular theses and dissertations. Brief quotations from this manuscript are allowable without special permission, provided that accurate acknowledgment of the source is made. Requests for permission for extended quotation from or reproduction of this manuscript in whole or in part may be granted by the Department of Geosciences when the proposed use of the material is in the interests of scholarship. In all other instances, however, permission must be obtained from the author. _______________________________________________ (author’s signature) ________________ (date) APPROVAL BY RESEARCH COMMITTEE As members of the Research Committee, we recommend that this prepublication manuscript be accepted as fulfilling the research requirement for the degree of Master of Science. __Karl W. Flessa________________________________ Major Advisor (signature) ________________ (date) __David L. Dettman_____________________________ Committee Member (signature) ________________ (date) __Andrew S. Cohen_____________________________ Committee Member (signature) ________________ (date) __Connie A. Woodhouse_________________________ Committee Member (signature) ________________ (date) 2 ABSTRACT Could stable isotopes of N be used to detect the source of nutrients in modern and past estuarine ecosystems? We examined δ15N from organic material contained in the shells of livecollected and sub-fossil bivalve mollusks from the Colorado River and its estuary. We compared δ15N in 3 species: Corbicula fluminea, an introduced freshwater species, Mulinia coloradoensis, a brackish-water species once abundant in the estuary, and Chione fluctifraga, a fully marine species. All three species are found alive today, and the latter two also occur in historical shell deposits. δ15N values obtained from modern C. fluminea are low (δ15N = 9.6 ‰) with respect to live-collected M. coloradoensis and Ch. fluctifraga (δ15N = 14.77 and 13.87, respectively), documenting a distinct difference between fluvial N signals and marine N signatures. We hypothesized that a significant difference in δ15N values would also exist between live-collected individuals and sub-fossil individuals of the same species. Riverine nutrients are largely absent from the modern Colorado River estuary because the river no longer reaches the sea, yet prior to dam construction and large-scale water diversions, such nutrients were likely available to estuarine biota. However, no significant difference in δ15N was found between modern and predam shells. To test for possible diagenetic distortion of the δ15N signal preserved in fossil shells, we subjected modern shells to conditions of strong heat (60oC in a drying oven) and intense sunlight (rooftop exposure in Tucson, Arizona) for 180 days. Whereas no significant directional shift in δ15N was detected in rooftop shells relative to controls, heated shells became depleted in 15 N. Diagenesis therefore cannot be ruled out as a source of bias in δ15N measurements. The similarity of δ15N in modern and pre-dam shells nevertheless suggests that either riverine nutrients were not generally a significant component of the estuarine nutrient pool or that their isotopic character was lost during biogeochemical transformations prior to incorporation into bivalve shells. INTRODUCTION Under natural conditions, estuaries function as sites of material exchange between continental and marine environments. Rivers deliver sediments and nutrients generated on the land, as well as an authochthonous component derived from aquatic primary production, to their deltas. Nitrogen transported in the fluvial environment enters the estuary in multiple forms, namely dissolved organic nitrogen (DON), dissolved inorganic nitrogen (DIN), and particulate organic nitrogen (PON). These “riverine nutrients” may then be used by estuarine biota. Tidal flushing and oceanic circulation mix and redistribute the river-derived materials among the marine nutrient pool. Freshwater flows and nutrient delivery to an estuary can vary over broad timescales in response to climatic shifts, geomorphologic processes (e.g. stream capture), and changes in landscape ecology (Correll et al. 1992; Spencer and Pearthree 2003; Jickells et al. 2000, Ingram et al. 1996). In recent times, the timing and quantity of river discharge and nutrient delivery has been altered by anthropogenic activities, including agriculture (water withdrawals, fertilizer runoff), urbanization (polluted stormflow, wastewater discharge, increased overland flow), vegetation clearance (reduced terrestrial organics), and damming and water diversions (diminished flow and suspended load). 3 It is important to document these changes because they can affect estuarine productivity and ecosystem composition (Mallin et al. 1993). Whereas recent changes can be observed and measured, historical variation can be discerned only indirectly from aquatic archives. Reconstructing former estuarine conditions requires the use of chemical and biological indicators preserved in sediments or the remains of organisms. In this study, we determine whether stable isotopes of nitrogen contained in the shells of bivalve mollusks record changes in riverine nutrient delivery to modern and past estuarine ecosystems. Suspension-feeding bivalves filter and assimilate phytoplankton, detritus, microzooplankton, and suspended organic sediments – and in some cases they additionally absorb dissolved organic material directly from water (Péquignat 1973; Manahan et al. 1982). Ferguson (1982) suggested that the uptake of dissolved free amino acids (DFAA) can support as much as 20% of bivalve metabolism, and Rice (1999) estimated that DFAA can account for up to 14% of total organic N uptake in the clams of Narragansett Bay, Rhode Island. Changes in the relative availability of these various nutrient types and their source regions (e.g. terrestrial, freshwater, or marine) are relevant to bivalve feeding and may be reflected as isotopic differences in their tissues and shells. Nitrogenous materials derived from terrestrial and marine sources possess distinct nitrogen isotope signatures (Sweeney et al. 1978; Sweeney and Kaplan 1980), with the latter being more enriched in 15N than the former. Fixation of atmospheric nitrogen (δ15N = 0 ‰) by land plants produces organic matter with δ15N values also near 0‰, typically between –3 ‰ and +1 ‰ (Kendall 1998). Partial degradation of these materials as they are transported downstream may raise their δ15N signatures some, but they typically remain strongly depleted relative to marine organic materials. Nitrogen-fixing phytoplankton (namely cyanobacteria) also generate organic matter with low δ15N and can be important in riverine environments. They are not abundant in the ocean, however, and usually comprise < 1 % of the phytoplankton biomass or they are absent altogether (Howarth et al. 1988). Marine N pools are strongly influenced by denitrification, which discriminates strongly against 15NO3- and drives the δ15N of residual N to high values (Liu and Kaplan 1989). The δ15N of PON is most relevant in our study, as this is the dominant fraction used by benthic consumers. Isotopic separation between marine and terrestrial suspended organic matter is often quite large. Marine end members for the δ15N of PON are geographically variable but generally are within the range of 6.3 ‰ to 10 ‰ (Liu and Kaplan 1989). Mariotti et al. (1984), using δ15N to trace the source of suspended PON in the Scheldt Estuary in northern Europe, applied a marine end member (North Sea) of 8 ‰ and terrestrial end member of 1.5 ‰ (Δ15Nmarine-terrestrial = 6.5 ‰). Wada et al. (1987) calculated a maximum Δ15N of 7 ‰ for marine and terrestrial primary producers in the Otsuchi River-Otsuchi Bay environment of Japan. Isotopic separation between only the autochthonous fraction of riverine and marine PON can also be large. Cloern et al. (2002) calculated mean δ15N values of 5.0 ‰ and 8.0 ‰ for freshwater and estuarine-marine phytoplankton, respectively, in the San Francisco Bay Estuary. Although the range of possible δ15N values for terrestrial, freshwater, and marine PON is large, these trends (terrestrial < freshwater < marine) appear to be widespread and systematic (France 1995). These distinct isotopic signals are also recorded in consumers, thus opening the possibility of using consumer δ15N values to determine local nutrient sources. Synthesizing the data available in the literature at the time, France (1994) determined that a mean separation of 3 ‰ existed between marine and freshwater zooplankton as well as marine and freshwater zoobenthos. Reanalyzing data from Peterson et al. (1985), he also determined that estuarine 4 mussels (Geukensia demissa) have δ15N values reflecting their position along a freshwatermarine gradient. Similarly, Kasai and Nakata (2005) showed a clear relationship between the δ15N of PON and the δ15N of Corbicula japonica foot tissue in different regions of the Kushida Estuary in Japan. PON in the Kushida River had a mean δ15N of 5.5 ‰ while marine PON was elevated at 9.0 ‰; estuarine PON δ15N was of an intermediate value (6.3 ‰). Co. japonica δ15N values increased from 8.6 ‰ in the upper estuary to 11.8 ‰ in the lower estuary, reflecting the physical mixing of nutrient sources and the increasing dilution of riverine PON down estuary. The offset between tissue and PON δ15N values, about 3.0 ‰, could be explained by normal trophic enrichment. Animal tissues are usually 2-5‰ enriched relative to diet (mean = 3.4‰, Minagawa and Wada 1984). Numerous other studies have reported δ15N values for bivalve soft tissues (see Table 1). Measurements of tissue δ15N, often complimented by measurements of δ13C, have been used to discern the relative importance of different dietary sources, to address spatial variability in available nutrient sources, and to track anthropogenic N pollution (see references within Table 1). Some investigators have also attempted to extract seasonal information from bivalve tissues harvested at different times of the year. Soft tissues cannot be used to study long-term environmental changes, however, because they are not preserved following the death of the organism. Bivalve shells, on the other hand, can serve as archives of paleoenvironmental information. Nitrogen contained within the organic material of bivalve shells may, like the N contained within soft tissues, record changes in bivalve diet and, thus, in sources of available nutrients. Shell organic matrices, which form prior to mineralization, are thought to provide a framework for crystalline growth (Bevelander and Nakahara 1969; Weiner and Hood 1975). Calcium carbonate and the organic constituents of the shell pass through mantle epithelia to the extrapallial fluid, where shell deposition occurs (Wilbur 1964; Neff 1972). While much of the organic material in the shell is contained within the “matrix” component, some of it becomes encased in the mineral crystals; this latter fraction is perhaps less likely to be affected by diagenesis and more likely to be preserved in fossil shells (Crenshaw 1980; O’Donnell et al. 2003). To our knowledge, only two studies have reported δ15N values for organic material within bivalve shells. O’Donnell et al. (2003) measured 15N (and δ13C, δ34S) in the soft tissues and shell material (ventral margin) of modern and sub-fossil Mercenaria spp. collected from the Atlantic coastal plain of the United States. Mae et al. (2007) measured 15N (and 13C) in both modern and fossil deep-sea bivalve shells for the purpose of evaluating whether historical populations utilized chemosynthetic production. In this study, we compare the nitrogen stable isotope composition of modern and sub-fossil bivalve shells collected from the Colorado River estuary. The Colorado River estuary serves as an ideal investigatory setting because the completion of massive dams on the Colorado River during the early-to-middle 20th Century all but shut down the inflow of river water. Biota in the modern “no-flow” estuary have access to only marine nutrients, but the same biota in the pre-dam estuary likely received a mixture of marine and riverine nutrients. The Colorado River would have transported terrestrial organic N and its degradation products, as well as an autochthonous component from freshwater primary producers, and possibly heterotrophic bacteria. We hypothesized that pre-dam bivalves would have “seen” these riverine nutrients and that their shells would therefore record a distinct (depleted) δ15N signature relative to shells collected from live individuals in the modern environment. In addition, we tested the hypothesis that the shells of freshwater bivalves 5 collected from several locations within the lower Colorado River drainage basin would be depleted in 15N relative to the shells of estuarine bivalves (both modern and pre-dam). Our analyses assume a constant isotopic offset between soft tissue and shell, such that any rise in the δ15N of ambient nutrients translates into higher shell δ15N and any decline in the δ15N of nutrient sources also reduces shell δ15N. Knowing the absolute value of that offset is not important for this study, but it may be critical in future investigations that aim to reconstruct historical trophic relationships. Hydrologic transformation of the Colorado River estuary following the cessation of flows off the river has affected many local fauna adversely (Aragón-Noriega and Calderon-Aguilera 2000; Galindo-Bect et al. 2000; Rowell et al. 2005), including bivalve mollusks and their predators (Rodriguez et al. 2001a; Cintra-Buenrostro et al. 2005). Kowalewski et al. (2000) estimated that the standing crop of bivalve mollusks, namely Mulinia coloradoensis and Chione fluctifraga, has declined by as much as 94% from an original size of about 6 billion individuals. It is unclear whether the disappearance of riverine nutrients has contributed to population declines. Despite the sharp reduction in consumer productivity, nutrient levels and primary productivity in the estuary remain high (Hernández-Ayón et al 1993; Millán-Núñez et al. 1999). We undertook this investigation not only with the aim to evaluate whether shell δ15N reflects the availability of nutrients derived from the Colorado River, but also with the objective to shed some light on whether riverine nutrients were important for benthic production in the pre-dam environment. Were riverine nutrients a significant component of the bivalve diet? METHODS Study site The Colorado River estuary is located between the Mexican states of Sonora and Baja California, at the terminus of the 2800-km-long Colorado River. Mean monthly air temperatures range from 16oC in winter and 34oC in summer, while mean monthly water temperature varies between 15oC and 30oC (Thompson 1968; Thompson 1999). The area receives less than 60 mm of annual rainfall (Hastings 1964), making local runoff into the estuary negligible (Dettman et al. 2004). The estuary lies within a shallow basin (1 to 40 m) and is characterized by a very large tidal range (up to 10 m) (Carbajal et al. 1997; Lavín and Sánchez 1999). Sediment resuspension by the strong tides generates turbid conditions (Hernández-Ayón et al. 1993; Santamaría-delÁngel et al. 1996), and the circulation of materials in the estuary generally follows a counterclockwise pattern (Carriquiry and Sánchez 1999). The completion of massive dams on the Colorado River during the early-to-middle 20th Century and subsequent diversion of enormous quantities of water profoundly altered the hydrological and ecological characteristics of the estuary. Prior to the dams and diversions, an unrestrained Colorado River annually discharged 8 x 109 to 30.8 x 109 m3 yr-1 of freshwater into the upper Gulf of California (Harding et al. 1995), creating a mixing zone that stretched at least 65 km southward from its mouth (Rodriguez et al. 2001b). For approximately the last 50 years, the river has rarely reached the Gulf, discharging only during unusually wet periods. Absent its flow, the modern Colorado River fails to deliver sediments and nutrients from the land to the sea. Delta wetlands have declined to a small fraction of their original area, oligohaline conditions in the upper estuary have vanished, and sediments are now eroded – not deposited – in intertidal areas (Lavín and Sánchez 1999; Thompson 1968; Carriquiry and Sánchez 1999). Cessation of 6 freshwater flows has introduced marine salinity conditions (35-42 psu) and inverted the normal salinity gradient of the estuary such that higher values occur toward the head (Alvarez-Borrego, 2001; Dettman et al. 2004). Sample collection Live specimens of Mulinia coloradoensis and Chione fluctifraga were collected in December 2005 from Estero del Chayo (31o40.12’ N, 114o41.53’ W), a tidal channel cutting into the deltaic sediments of Isla Montague (Fig. 1). Both species are infaunal suspension feeders native to the Colorado River estuary. Whereas Mulinia prefer brackish conditions (which are today absent), Chione can be considered fully marine. In the absence of inflow from the Colorado River, shells from these individuals are presumed to represent marine conditions. To obtain a representation of the modern fluvial environment, Corbicula fluminea were collected from multiple locations in the Lower Colorado River drainage. C. fluminea is an introduced freshwater species that colonized the lower Colorado River only after the completion of the major upstream dams. Live individuals were collected from Mitry Lake and Imperial Lake (December 2004), and from a section of river flowing through the city of Yuma, AZ (April 2007). The shells of recently expired individuals were obtained from Lake Mead (March 2008) and Walter’s Camp and Island Lake (November 2007). In addition, two unionid mussels were obtained – one from Mitry Lake (live collected, December 2004) and one from Smithsonian archives (shell only, collected in the 1880s from the Colorado River at the Mexican border). All live-collected specimens were kept in cold storage until preparation for isotopic analysis. Mitry Lake and Island Lake are small, shallow backwaters connected to the main river channel. Walter’s Camp, located on the river, is a privately owned concession in the Cibola National Wildlife Refuge. Each of these 3 sites is found within a short distance (<50 km) to the north of Yuma, as is Imperial Lake, a major impoundment formed by the Imperial Dam. Lake Mead, formed by waters impounded by Hoover Dam, is located much further north, near Las Vegas. Subfossil shells of Mulinia and Chione were collected from cheniers, or long shelly beaches, on the western shores of the estuary. Amino acid racemization and radiocarbon dates (Kowalewski et al. 1994, 1998) indicate that the shells in these locations were produced by bivalves living prior to the construction of upstream dams. Oxygen isotope data (Rodriguez et al. 2001b; Dettman et al. 2004) confirm that the bivalves were influenced by freshwater inflows off the Colorado River. Pre-dam Mulinia specimens used in this study were obtained from a chenier at Las Isletas (December 2001); pre-dam Chione were collected at a chenier on Isla Sacatosa (November 2005) (Fig. 1). All shells were obtained from > 25 cm depth to minimize any diagenetic effects from surface exposure. Sample preparation All shells were thoroughly cleaned and rinsed prior to taking δ15N measurements. Soft tissues were removed with a scalpel from live-collected bivalves. Both live-collected and predam shells were scrubbed with a stiff nylon brush to remove attached dirt and debris. The periostracum, an organic coating on the exterior shell surface that can differ isotopically from shell material (unpublished data), was removed using a dental drill. Most pre-dam shells appeared to have lost their periostracum, but the exterior shell surface was still cleaned with the 7 drill to be certain that all of the coating had been removed. Shell surfaces were rinsed with deionized water after brushing and drilling to remove loosened material. One valve of each shell was approximately bisected using a low-speed ISOMET lapidary saw with diamond-tipped wafer blade. One of these halves was then ground into a powder and homogenized using a mortar and pestle (Fig. 2). δ15N measurements on these “whole-shell” powders reflect the average isotopic composition of shell material over the lifetime of the bivalve. Some Chione shells were too thick to grind with a mortar and pestle; in such instances a cross-sectional slice (about 2-3 mm wide) was cut along the axis of maximum growth between the umbo and ventral margin. The slice was then easily ground with mortar and pestle. Powders obtained from ground cross-sections should be equivalent to “whole-shell” powders in their average isotopic composition. For some bivalve samples, shell material was additionally obtained from the ventral margin of the other valve half. Drilling at the margin produced a fine powder that did not require further grinding with a mortar and pestle. δ15N measurements on “margin” powders reflect the isotopic composition of the most recently deposited shell material only. The remaining valve was, for some individuals, spot sampled along a vertical transect between the umbo and ventral margin. Shallow circular drill holes were made at semiregular intervals in the exterior shell surface to extract small amounts of powder for carbonate δ18O measurements. The δ18O data were then used to identify portions of the shell deposited during early summer, which, in the pre-dam environment, is when the strongest freshwater flows off the Colorado River would have occurred (Harding et al. 1995). These summer growth bands were subsequently sampled for δ15N measurements. Isotope measurement For δ15N measurements, homogenized shell powder, usually in a quantity between 40 mg and 90 mg, was loaded into tin capsules. Sample capsules were combusted using a Costech elemental analyzer coupled to a Finnigan Delta Plus XL continuous-flow gas-ratio mass spectrometer. δ15N values were reported with precisions between + 0.1 ‰ and + 0.5 ‰ (1σ), as the amount of sample powder available for analysis was not always sufficient to obtain maximum precision. For simplicity, a standard error of + 0.5 ‰ should be assumed for all δ15N values reported in this study. Mass spectrometer standardization is based on IAEA-N-1 and IAEA-N-2 for δ15N. Shell carbonate δ18O was measured using an automated carbonate preparation device (Kiel-III) coupled to a Finnigan MAT 252 gas-ratio mass spectrometer. Powdered samples were reacted with dehydrated phosphoric acid under vacuum at 70oC. The isotope ratio measurement is calibrated based on repeated measurements of NBS-19 and NBS-18 and precision is + 0.1 ‰ for δ18O (1σ). Diagenesis experiment To test for possible diagenetic distortion of the δ15N signal preserved in pre-dam shells, we subjected a subset of modern shells to conditions of strong heat and intense sunlight – the most likely causes of any diagenetic effects – for ½ year, between April 4, 2007 and September 29, 2007. For each shell (n=10; 4 Corbicula, 3 Mulinia, and 3 Chione), one valve was used for experimentation and one valve was kept aside as a control. The experimental valve was bisected using the low-speed lapidary saw; one half was then placed in a drying oven set to a constant 8 temperature of 60oC and the other was rested upon a rooftop in Tucson, Arizona to receive maximum sunlight exposure. A thin wire meshing was fixed over the rooftop shells to prevent translocation by wind or animals, and a HOBO® datalogger placed 10 cm away recorded local temperature and light intensity beginning on April 19. Maximum daily temperatures frequently exceeded 60oC after April (Table 2) and averaged < 40oC. Maximum daily light intensity between 1.6 x 105 and 2.3 x 105 lux were common. RESULTS Fluvial vs. marine δ15N signals To be able to interpret temporal δ15N shifts (pre-dam vs. modern Mulinia and Chione) in terms of changes in riverine nutrient delivery, we first needed to establish whether fluvial and marine δ15N signals are indeed distinct in the area of investigation. For this, we compared the shells of Corbicula collected from portions of the lower Colorado River drainage to the shells of modern estuarine clams, which have been subject to only marine conditions since the starvation of river flow. Mean whole-shell δ15N values for Corbicula at each sampling site (Table 3) range from 4.88 ‰ (Mitry Lake) to 12.51 ‰ (Lake Mead). Reasons for this extremely large range are postulated in the discussion section. Each site mean is lower than the mean whole-shell δ15N values obtained for modern Mulinia and Chione (13.87 ‰ and 14.77 ‰, respectively; Table 4). Likewise, the overall location-based mean for Corbicula (9.55 ‰) is far lower than the marine signal obtained from Mulinia and Chione. If each sampling location is not given an equal weight and a mean is instead constructed from all Corbicula shells, regardless of site, the value falls slightly to 9.04 ‰. In addition, 2 unionid mussels, 1 from Mitry Lake and the other from a stretch of river at the international border, record depleted δ15N signals (whole-shell values < 6 ‰), which further illustrate that bivalves in the fluvial environments of the Colorado River are isotopically distinct (lighter) from bivalves in the marine conditions of the modern Colorado River estuary. Modern vs. Pre-dam δ15N signals Whole-shell δ15N values for modern Mulinia exhibit a fair amount of variability (std . dev. = 1.11) and range from 11.49 ‰ to 14.71 ‰. The overall whole-shell mean equals 13.87 ‰. This differs slightly from the whole-shell mean of pre-dam Mulinia (12.14 ‰). The predam mean is pushed downward by shells D11 and D12, which possess anomalously low wholeshell values (7.85 ‰ and 7.96 ‰, respectively) compared to all other whole-shell data for Mulinia and Chione. If these two values are removed, the pre-dam whole-shell mean becomes 13.22 ‰ and the remaining values show a standard deviation of 0.74. However, even using the full dataset, pre-dam and modern populations of Mulinia do not show statistically significant differences in whole-shell δ15N (1-way ANOVA, p = 0.406). Whole-shell δ15N values for modern Chione range from 14.26 ‰ to 15.43 ‰ and are fairly consistent (std. dev = 0.42). The overall whole-shell mean equals 14.77 ‰. Pre-dam values are more variable (std. dev = 1.46) and range from 12.41 ‰ to 16.10 ‰. The whole-shell mean is nearly identical to that of modern Chione, however, and there is no statistically significant difference between the two populations (p = 0.760). Comparisons between species (not across time) reveal statistically significant differences. Whole-shell δ15N values for Mulinia 9 were consistently lighter than for Chione (p= 0.006 for modern shells; p = 0.031 for pre-dam shells) Intra-shell δ15N variation Table 5 displays multiple series of δ18O values obtained from drill samples of surface carbonates in the shells of 3 pre-dam Mulinia (D10-D12), 3 pre-dam Chione (G1-G3), 1 modern Mulinia (C4), and 1 modern Chione (B4). Pre-dam δ18O series include much more negative values than modern δ18O series, reflecting the presence of Colorado River water (Dettman et al. 2004). The lowest values in each of the pre-dam bivalve profiles, highlighted in the Table 5 and boxed in Fig. 3, are interpreted to represent the early summer growth period (approx. May through July), when strong pulses of snowmelt produced most of the Colorado River’s annual flow (Harding et al. 1995). Because the δ18O profiles of modern bivalves are not affected by river flow, they record a simple temperature cycle. The lowest values mark shell growth during the warmest part of the year (i.e. summer). Once identified, the summer shell bands in both pre-dam and modern bivalves were drilled for 15N measurements. In a few instances, marked differences in δ15N between summer shell bands and whole-shell material are apparent, but these differences are not consistent in sign. Although the summer-shell δ15N means reported in Table 4 for each bivalve population are less than corresponding whole-shell means, they are based on fewer data points and may be biased toward shells that, for whatever reason, have lighter isotopic values than the “average” bivalve within the population. It is therefore more useful to turn to Table 6, which compares summershell δ15N values and whole-shell δ15N for individual bivalve specimens. These data show no obvious relationship between the nitrogen isotopic signatures of whole-shell and summer-shell material. In addition, there is no statistically significant difference between the pre-dam wholeshell values and pre-dam summer shell values for either species (Mulinia, p = 0.406; Chione, p = 0.760) Interestingly, δ15N values obtained from shell material at the ventral margin are, with a single exception, lighter than whole-shell values, regardless of bivalve species. In many cases, there is a wide separation (> 3 ‰) between margin and whole-shell values, particularly in modern Mulinia. There is a statistically significant difference between margin δ15N and wholeshell δ15N for modern Mulinia (p = 0.007) but not for modern Chione (p = 0.257). Diagenesis experiment δ15N values obtained from shells placed on a Tucson rooftop for approximately ½ year were similar to δ15N values obtained from controls. Differences in isotopic signature were minor and bi-directional in sign (Table 7, Fig. 4), suggesting that preferential degradation of specific N species within the shell had not occurred. Data obtained from shells placed in the drying oven appear to indicate a diagenetic shift toward lighter values, as the difference in δ15N between controls and oven samples was, with only a single exception, always negative in sign. This suggests that heating may be more important than sunlight exposure in terms of its diagenetic effect on stable isotopes of N. The δ15N of pre-dam estuarine bivalve shells could have been shifted toward lighter values due to heat-related diagenesis. 10 DISCUSSION Fluvial vs. marine δ15N signals Whole-shell δ15N values for Corbicula varied strongly between sampling locations; this may reflect differences in denitrification rates, anthropogenic N inputs, and/or other environmental factors. Yuma and Walter’s Camp are the only sites where Corbicula were collected from the Colorado River proper (not a deep artificial lake or off-channel backwater lake), and δ15N means from these locations (9.60 ‰ and 9.52 ‰, respectively) are nearly identical. Island Lake, located between these two sites, produced shells with a similar signature (9.88 ‰). Island Lake and Walter’s Camp are located in sparsely populated areas north of the city and are not likely influenced by wastewater discharges or other anthropogenic N inputs. The absence of an enriched δ15N signal in the Yuma shells suggests that the collection site is located upstream from the city’s major effluent releases. δ15N values from these 3 sites may best represent the pre-dam fluvial N signature. High δ15N values from Lake Mead shells were not surprising, since the shells were collected in the lake’s western basin, which receives wastewater from Las Vegas. Human wastes (and other animal wastes) are enriched in 15N and generally have an isotopic composition between +10 to +20 ‰ (Kendall 1998; McClelland et al. 1997). Denitrification in deep sediments may have also contributed to an enriched δ15N signal. No major wastewater inputs to Imperial Lake are known; intermediate δ15N values obtained from shells collected at this site likely reflect isotopic enrichment from denitrification. The reasons for much lower δ15N values in Mitry Lake shells are unclear. It is notable that whole-shell δ15N means obtained from bivalves at each sampling location in the lower Colorado River drainage – even locations with enriched δ15N signals due to wastewater inputs and higher levels of denitrification – are less than whole-shell δ15N means obtained from bivalves living in the modern Colorado River estuary. This supports the assumption that riverine nutrients are isotopically distinct (lighter) from marine nutrients. Interestingly, the mean whole-shell δ15N values for modern Mulinia and Chione differ by almost 1 ‰. Local habitat variation does not explain this difference because individuals of both species were collected from the same sample site. This instead suggests species-specific differences in feeding strategy. For example, one species may filter a specific size class of particles more efficiently than the other. Modern vs. Pre-dam δ15N signals Riverine nutrients may be acquired directly or indirectly by estuarine bivalves. PON generated in the river (freshwater phytoplankton, small zooplankton, and bacteria) may pass into the estuary and be filtered out of suspension. Likewise, PON produced on land but transported by the river (terrestrial detritus) may reach the estuary and be removed from the water column. Dissolved inorganic nutrients (NO3-, NH4+) from the river cannot be acquired directly, but they may be utilized by estuarine phytoplankton, which are then filtered by the bivalve. DON may be obtained either directly (absorbed) or indirectly (first consumed by estuarine bacterioplankton or phytoplankton). Given these several modes of riverine nutrient utilization and the strong separation of fluvial and marine isotopic signatures discussed above, it is surprising to find an absence of significant difference between whole-shell δ15N values in the modern and pre-dam 11 estuarine bivalve populations. The similarity of δ15N in modern and pre-dam shells presents several possibilities: 1) Riverine nutrients were not a significant component of the total nutrient pool utilized by estuarine bivalves; 2) Riverine nutrients represented a sizeable fraction of the total nutrient pool but lost their distinctive (depleted) isotopic signature during biogeochemical transformation; or 3) Depleted signals from riverine nutrients were recorded in the shell but counterbalanced by processes leading to isotopic enrichment 1. “Riverine nutrients were insignificant” Page & Lastra (2003) found no evidence from 15N and13C measurements on bivalve stomach contents and muscle tissue that river-derived PON was a significant portion of bivalve diets in the Ría de Arosa estuary of northwest Spain. δ15N values did not track distance from riverine resources. This estuarine system, however, is marine-dominated and freshwater inputs are small compared to tidal exchange. The pre-dam Colorado River estuary experienced much stronger freshwater flows. Unless strongly affected by anthropogenic N loading, most rivers do not contain high concentrations of DIN, and a significant portion of what does reach the mouth can be intercepted by coastal wetlands (Schlesinger 1997). The intercepted N may be assimilated into plant biomass, recycled within the wetland, removed via sedimentation or denitrification, or exported in dissolved and particulate fractions. N losses due to denitrification are equivalent to 40-50 % of the DIN entering from rivers (Seitzinger 1988). Organic forms generally dominate the total nitrogen load transported by rivers (Ittekot and Zhang 1989; Seitzinger & Sanders 1997; Scott et al. 2007). Some particulate organics may settle in the wetlands as the velocity of surface waters slows, but tidal action can be expected to mobilize some material and send it into the open waters of the estuary. Overall, tidal flushing results in a net movement of nutrients away from coastal marshes and toward the open estuary (Valiela and Teal 1979; Correll 1981; Nixon 1980). We therefore assume that a significant quantity of nutrients were flushed into the pre-dam Colorado River estuary. Moreover, because the wetlands in the delta were far more expansive than today, it is likely that more organic N was produced within these environments and delivered to the open estuary. The presence of nutrients does not necessarily guarantee their biologic availability, however. If too refractory, nutrients cannot be metabolized. Suspension-feeding bivalves separate particles with low nutritional value, such as refractory detritus, and deposit them as pseudofeces (Kiørboe and Mohlenberg 1981). According to Fry (1999), most riverine seston entering the North Bay of San Francisco is refractory, and it comprises only a minor portion of the diet of suspension feeders. Canuel et al. (1995) generalize that the pool of organic matter in estuaries consists primarily of refractory components. In the Miya Estuary of Japan, for example, 90% of PON originates from terrestrial materials and yet this fraction represents only 10% of the diet of resident bivalves (Ruditapes philippinarum and Mactra veneriformis) (Kasai et al. 2004). Much of the DON that reaches estuarine environments is also refractory, and its resistance to microbial attack favors its passage through coastal marshes and into the open waters of the estuary (Valiela and Teal 1979). Unlike the labile, low molecular weight compounds (e.g. 12 amino acids and urea), which generally represent less than 20% of the riverine DON (Thurmann 1985), these components may not be readily utilized by the local biota. 2. “Riverine nutrients lost their depleted δ15N signatures” Biogeochemical transformations of nitrogenous materials in estuaries and their adjacent wetlands can exert strong influence over δ15N signals, sometimes to a greater extent than the physical mixing of riverine and marine nutrient sources (Cifuentes et al. 1988; Sato et al. 2006) δ15N may vary spatially and temporally within these environments due to changes in the rate/amount of phytoplankton N uptake and microbially-mediated N conversions (i.e. nitrification, denitrification) (Montoya et al. 1990). Isotopic fractionation during assimilation by phytoplankton depends on N availability, the chemical form that is utilized, and the species of phytoplankton (Waser et al. 1998), and the range of potential fractionation values is quite large (Needoba and Harrison 2004; Waser et al. 1999). In the Sumida River Estuary of Japan, seasonal variations in the δ15N of suspended PN were attributed to changes in the taxonomic composition of phytoplankton (Sato et al. 2006). Denitrification in coastal marshes and estuarine sediments is important not only because it removes some riverine nutrients (as discussed above) but also because it raises the δ15N of residual N moving into the water column of the estuary (Valiela and Teal 1979). Producers utilizing this 15N-enriched nutrient source then become isotopically enriched themselves. Nitrification has the opposite effect – it creates a pool of 15Ndepleted nitrate – but nitrification and denitrification are often coupled processes, such that nitrates generated via the former support the latter (Seitzinger 1988; Kemp et al. 1990). In addition, nitrification has the effect of increasing the δ15N of residual ammonium. Because phytoplankton preferentially utilize ammonium over other DIN fractions (Glilbert et al. 1982; Dortch 1990; L’Helguen et al. 1996), this can mean that the enriched δ15N signal is transferred to phytoplankton and upward to consumers. Mariotti et al. (1984) found that nitrification in the Scheldt Estuary produced higher δ15NNH4 values downstream and argue that the isotopic signature of suspended organic matter, which also becomes more enriched downstream, responds to nitrification. If these various biogeochemical transformations cause a net increase in the δ15N of DIN, and if primary producers utilizing this enriched nutrient source constitute a significant fraction of consumer diets, local bivalves will record higher δ15N values than they would if no biogeochemical alteration took place and only physical mixing of riverine and marine nutrient sources mattered. 3. “Depleted signals were counterbalanced by other factors” Any process or influence which raises the δ15N values of pre-dam shells or lowers the δ N values of modern shells would reduce the expected temporal contrast in δ15N between the two bivalve populations. 15 Ontogeny We assume that ontogenetic changes do not influence the nitrogen isotopic composition of adult bivalves. Minagawa and Wada (1984) concluded that the δ15N of soft tissues in two species of marine mussels, Septifer virgatus and Mytilus edulis, is independent of both age and internal nitrogen mass balance – except during “early stages” of growth. Other studies have 13 noted δ15N offsets between juveniles and adults of the same species (Kang et al. 1999; O’Donnell et al. 2003), perhaps due to the utilization of different food resources. However, all isotope measurements in this study were performed on adult individuals. Anthropogenic nitrogen loading Agricultural return flows and sewage effluent supply a very minor quantity of water and nutrients to the modern Colorado River estuary. Animal wastes are also minimal. We do not expect these inputs to influence the marine nutrient signal preserved in modern bivalve shells. Any anthropogenic influences on nutrient types or levels in the pre-1930 estuary should likewise have been small. Diagenesis The organic matrix of bivalve shells is composed of a complex assemblage of proteins, glycoproteins, polysaccharides, and lipids (Addadi 2006). Selective degradation of one of these components, or a subset of one of these components (e.g. specific amino acids), could potentially produce a shift in δ15N. Vallentyne (1969) measured differential decomposition rates for amino acids in Plesitocene Mercenaria shells during pyrolization at 174-252oC. He observed the following order of amino acid stability (from least to greatest): tyrosine < phenylalanine < isoleucine < leucine < proline < valine < glycine < alanine < glutamic acid. Risk et al. (1997), in a nuclear magnetic resonance spectral analysis of modern and fossil bivalve shells (Mya truncata, Hiatella arctica, Macoma baltica, and Mya arenia), found that Chitin, a polysaccharide, undergoes breakdown early in diagenesis relative to other components, and analyses on Mya truncata suggested selective degradation of some amino acids over others. Spatial heterogeneity in the distribution of nitrogenous compounds may also lead to diagenetic δ15N shifts if degradation processes favor one region of the shell over another. Goodfriend et al. (1997) found that the concentration of amino acids in each of the 3 structural layers of Ch. fluctifraga differed, with the middle layer containing the lowest concentration and the inner layer containing the highest. In addition, diagenesis may occur more rapidly in thin shells than in thick shells (Risk et al. 1997). Mulinia have much thinner shells than Chione, but the data obtained in our diagenesis experiment do not appear to indicate a more pronounced isotopic shift in one species relative to the other. Our data do suggest that diagenetic alteration of δ15N signals in the shells of pre-dam Mulinia and Chione is possible and that such alteration is more likely attributable to warm temperatures rather than sunlight exposure. Shells heated in a drying oven at 60oC for a half year are characterized by lower δ15N values than controls, while shells resting upon a sunlit rooftop for the same period showed no obvious directional shift in δ15N values relative to controls. However, δ15N values obtained from shell M4 (Corbicula) may indicate diagenetic effects from sunlight exposure. The shell was collected from a paleoshoreline of Lake Mead. One valve, fully buried in sediment, retained all of its periostracum while the other valve, partly exposed, had lost most of its periostracum, thus suggesting differential degradation of the two valves. After cleaning in the laboratory and complete removal of the periostracum, each valve was separately ground into a powder and homogenized. The δ15N value obtained from the exposed valve was over 1 ‰ lighter than the value obtained from the buried valve. Pre-dam Mulinia and Chione shells were collected from below 25 cm depth and were completely buried by other 14 shells. Differential sunlight exposure would not have occurred and total exposure prior to burial was probably minimal. We observed that more shell material was needed for pre-dam shells than for modern shells to obtain precise δ15N measurements on the mass spectrometer. This implies nitrogen loss from the shell with age. Similarly, O’Donnell et al. (2003) found a 50-70% decrease in the concentrations of both carbon and nitrogen in Pleistocene Mercenaria shells relative to modern shells. They warn that this reduction could amplify the influence of any contaminants. Thus our results confirm the possibility that this N loss bears an isotopic effect and suggest that prolonged heating can lead to lighter δ15N values, but it is not possible to ascertain based on available data whether the δ15N signatures of the pre-dam bivalves sampled in this study were, in fact, altered by diagenesis. If diagenetic effects were significant, they would have likely lowered δ15N values and increased the isotopic contrast between pre-dam and modern shells. Because we observe little to no temporal contrast in δ15N values in the first place, diagenesis cannot be used to explain the “missing” riverine signal. Intra-shell δ15N variation In the absence of strong evidence from whole-shell δ15N measurements for isotopically distinct pre-dam and modern bivalve populations, we sought to identify whether summer shell deposits recorded unique δ15N values that were masked by whole-shell averaging. Most of the Colorado River’s annual flow occurs between May and July due to strong pulses of snowmelt in the upper basin (Harding et al. 1995). For the San Pedro River, an arid-zone tributary to the Colorado River, Brooks et al. (2007) showed that large flood pulses following summer monsoon storms can produce substantial increases in the flux and concentration of both dissolved and particulate organic matter transported downstream. In a subestuary of Chesapeake Bay, Correll (1981) found that increased exports of total nitrogen from intertidal marshes into the estuarine basin were synchronous with periods of high flow from the Rhode River. The period of greatest discharge of the pre-dam Colorado River may therefore be expected to bring the greatest quantity of riverine nutrients to the estuary relative to marine sources. Whole-shell δ15N values for pre-dam bivalves represent the average of all organic material deposited in the shell over the lifetime of the individuals, during flood periods and lowflow periods alike. Because a greater total quantity of shell material is deposited during lowflow periods, whole-shell averages may obscure the brief utilization of low-δ15N nutrients during the flood season. Given this possibility, we constrained the portions of bivalve shells deposited during peak river flow and measured the δ15N organic material within these specific shell zones. Whole-shell and flood-pulse values in Chione, for both pre-dam and modern shells, are fairly similar (< 1‰ separation). Results from pre-dam Mulinia show variable isotopic separation between whole-shell values and summer values and do not necessarily indicate greater riverine nutrient utilization during flood periods. In the pre-dam Mulinia shell D10, a surprisingly large isotopic separation (4.2 ‰) was observed between the whole-shell value and summer value. Because the summer value is lower, it may reflect the incorporation of riverine nutrients. However, in shells D11 and D12, measured whole-shell δ15N values are more depleted relative to flood-pulse values. This is difficult to interpret, particularly since the whole-shell values are anomalously low compared to all other whole-shell values obtained from both modern and predam Mulinia. The low whole-shell values, regardless of how they compare to flood-pulse values, suggest that riverine nutrients reached the bivalves for the majority of their lives. Did 15 these two shells grow during exceptionally wet years? δ18O profiles for D11 and D12 carbonates do not seem to support this interpretation, as they do not differ substantially from the δ18O profile for D10, which has a more “typical”δ15N value. However, more pronounced seasonal minima in 18 O are apparent for D11 and D12. Taken together, the results from these 3 shells are ambiguous. Measurements on additional pre-dam Mulinia shells may reveal a significant trend that is not apparent from our limited data. Our sample size is too small to reveal the true complexity and variability of this dynamic ecosystem. A single modern Mulinia shell (C4) was also examined and showed negligible isotopic separation between whole-shell and summer values, which is what we expected for the present no-flow environment. The absence of a clear riverine nutrient signal in the summer growth bands of the sampled shells may be due to slow rates of tissue turnover. Because the shell organic matrix is secreted by mantle epithelia (Wilbur 1964; Neff 1972), its isotopic composition may be subject to the same temporal averaging as internal soft tissues. Table 8 summarizes published rates of tissue turnover in bivalves (Unfortunately, the turnover rates of mantle tissue have not been investigated.) These long turnover rates imply that shell δ15N does not record transient conditions but rather long-term average conditions. It may therefore be unreasonable to expect shell organics deposited during flood periods to bear distinctly lower δ15N signatures. This also suggests that fine-resolution sampling of changes in diet (and of nutrient source) may not be possible. Moreover, δ15N measurements (unlike δ13C and δ18O) require large quantities of shell material, often upwards of 30 mg, which precludes sampling within very narrow growth bands. In northeastern freshwater lakes, McKinney et al. (2002) found that the δ15N of Unionidae mussel (Elliptio spp.) tissues correlated with long-term nitrate concentrations but not with nitrate concentrations measured on a single sampling date. This, they argue, suggests that mussels act as long-term integrators of the δ15N of primary producers and that they do not record transient shifts. Gustafson et al. (2007) also assert that bivalve tissues cannot be used as proxies for pulse events or rapid changes in DIN loads. On the other hand, some studies have shown significant short-term changes in the δ15N of bivalve soft tissues (Goering et al. 1990; Fry and Allen 2003; Piola et al. 2006). Our own data from other portions of bivalve shells seem to suggest sub-annual δ15N changes. δ15N values obtained from the ventral margins of the bivalve shells seem to be lighter than whole-shell δ15N values in most instances. Because all estuarine bivalve samples were collected in late November or early December, shell margin material – which represents the most recent growth – was likely deposited just prior to winter growth shutdown (Mulinia and Chione generally cease to grow for several months during winter because of low temperatures (Goodwin et al. 2001). River flow is not substantial at this time of year, suggesting that the shift to lighter δ15N is controlled by factors unrelated to freshwater flow. If the deposition of organic material in bivalve shells responds to transient conditions, sub-annual changes in δ15N may reflect seasonal variability in the size of nutrient pools and the rates of biogeochemical transformations in the estuary and surrounding wetlands (e.g. Kaplan et al. 1979; Cifuentes et al. 1988; Sato et al. 2006). Reduced denitrification in winter may help to explain lower margin δ15N values and, in some cases, higher summer values (where they occur). Rates of denitrification vary as a function of temperature (Jorgensen 1989; Kaplan et al. 1979). In the Tama estuary of Japan, for example, Nishio et al. (1983) found that denitrification rates increased by a factor of four between December (9.3oC) and May (18.0oC). Reduction of dissolved oxygen availability in summer may also promote increased denitrification during summer. York et al. (2007) noted higher δ15N of ammonia during summer in the Waquoit Bay 16 estuary of Massachusetts, which they attribute to more rapid biologic processing of the nutrient; they also found higher δ15N of phytoplankton. In the Scheldt estuary of northen Europe, Mariotti et al. (1984) measured sharp increases in the δ15N of suspended PON. Whereas in winter, the estuary showed a clear continental-to-marine gradient in the δ15N (most suspended PON during this season was from the mixing of terrestrial and marine sources), summer δ15N values were high in both the upper and lower estuary (due to strong authochthonous production that utilized enriched ammonium generated by nitrification). Seasonal differences in circulation and upwelling in the Gulf of California are probably unimportant. The northern Gulf is shallow (< 200m) and partially separated from the main gulf by a sill and midrift islands (Santamaría-delÁngel et al. 1994; Pagau et al. 2002). The extent to which southern currents and upwelling nutrients seasonally affect the northern Gulf is unclear. SUMMARY A distinct difference between the whole-shell δ15N signatures of freshwater bivalves and their estuarine-marine counterparts suggests that bivalve shells can be used to determine historical nutrient sources and changes in the availability of a particular class of nutrients over time. However, we found no clear evidence of a riverine nutrient signal in the shells of bivalves that lived in the pre-dam Colorado River estuary. It may be that riverine nutrients were not a significant component of the total nutrient pool in the pre-dam estuary. Alternatively, biogeochemical modification of riverine nutrients in the estuarine environment and its fringing marshes could have diminished the isotopically-distinct (depleted) riverine nutrient signal. Other processes that might “mask” a riverine nutrient signal (by raising δ15N) are probably insignificant. We determined that heat-related diagenesis can reduce the δ15N of older shells, but a diagenetic shift toward lighter δ15N values would only lead to overestimation of riverine nutrient utilization. Because we find no clear evidence of a riverine nutrient signal, the possibility of diagenesis did not influence our analysis. The results of this study do not support the use of whole-shell δ15N signatures in estuarine bivalves as an indicator of riverine nutrient availability. However, observed sub-annual differences in shell δ15N raise the possibility of detecting short-term changes in estuarine conditions. Better understanding of intrashell δ15N variability, tissue turnover time, and the relationship between soft tissues and shell organics is needed. ACKNOWLEDGEMENTS We thank Majie Fan and Chris Eastoe for processing our sample materials in the Environmental Isotope Laboratory and for providing helpful suggestions on sample preparation. We are also grateful to the fishermen of El Golfo de Santa Clara for transporting us to clam collection sites in the Colorado River Delta. The Smithsonian Institute provided an 1880s unionid mussel collected from the lower Colorado River, on which we conducted δ15N measurements. This research project was supported by the National Science Foundation (Grant No. 044381, awarded to KWF) and the Colorado River Delta Research Coordination Network. RDD received an Exxon Scholarship and Geosciences General Scholarship (University of Arizona) and was provided additional support through the Bert S. Butler Fund and Charles Evenson Fund. 17 REFERENCES Addadi, L., D. Joester, F. Nudelman, and S. Weiner. 2006. Mollusk shell formation: A source or new concepts for understand biomineralization processes. Chemistry – A European Journal 12: 980-987. Alvarez-Borrego, S. 2001. The Colorado River estuary and upper Gulf of California, Baja, Mexico. In U. Seeliger and B. Kjerfve [eds.], Coastal Marine Ecosystems of Latin America. Ecological Studies 144. Springer-Verlag. Aragón-Noriega, E.A. and L.E. Calderon-Aguilera. 2000. Does damming the Colorado River affect the nursery area of blue shrimp Litopanaeus stylirostris (Decapods: Penaeidae) in the upper Gulf of California? International Journal of Tropical Biology and Conservation 48: 867-871. Bevelander, G. and H. Nakahara. 1969. An electron microscope study of the formation of the nacreous layer in the shell of certain bivalve mollusks. Calcified Tissue Research 1969: 84-92. Bouillon, S., A.V. Raman, P. Dauby, and F. Dehairs. 2002. Carbon and nitrogen stable isotope ratios of subtidal benthic invertebrates in an estuarine mangrove ecosystem (Andhra Pradesh, India). Estuarine, Coastal and Shelf Science 54: 901-913. Brooks, P.D., P.A. Haas, and A.K. Huth. 2007. Seasonal variability in the concentration and flux of organic matter and inorganic nitrogen in a semiarid catchment, San Pedro River, Arizona. Journal of Geophysical Research 112: G03S04 Brusati, E.D. and E.D. Grosholz. 2007. Effect of native and invasive cordgrass on Macoma petalum density, growth, and isotopic signatures. Estuarine, Coastal and Shelf Science 71: 517-522. Bucci, J.P., S. Rebach, D. DeMaster, and W.J. Showers. 2007. A comparison of blue crab and bivalve δ15N tissue enrichment in two North Carolina estuaries. Environmental Pollution 145: 299-308. Cabana, G. and J.B. Rasmussen. 1996. Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Sciences 93: 10844-10847. Canuel, E.A., J.E. Cloern, D.B. Ringelberg, J.B. Guckert, and G.H. Rau. 1995. Molecular and isotopic tracers used to examine sources of organic matter and its incorporation into the food webs of San Francisco Bay. Limnology and Oceanography 40: 67-81. Carbajal, N., A. Souza, and R. Durazo. 1997. A numerical study of the ex-ROFI of the Colorado River. Journal of Marine Systems 12: 17-33. Carmichael, R.H., A.C. Shriver, and I. Valiela. 2004. Changes in shell and soft tissue growth, tissue composition, and survival of quahogs, Mercenaria mercenaria, and softshell clams, Mya arenia, in response to eutrophic-driven changes in food supply and habitat. Journal of Experimental Marine Biology and Ecology 313: 75-104. Carriquiry, J.D. and A. Sánchez. 1999. Sedimentation in the Colorado River Delta and upper Gulf of California after nearly a century of discharge loss. Marine Geology 158: 125-145. Cifuentes, L.A., J.H. Sharp, and M.L. Fogel. 1988. Stable carbon and nitrogen isotope biogeochemistry in the Delaware Estuary. Limnology and Oceanography 33: 1102-1115. Cintra-Buenrostro, C.E., K.W. Flessa, and G. Avila-Serrano. 2005. Who cares about a vanishing clam? Trophic importance of Mulinia coloradoensis inferred from predatory damage. Palaios 20: 296-302. 18 Cloern, J.E., E.A. Canuel, and D. Harris. 2002. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay Estuarine System. Limnology and Oceanography 47: 713-729. Correll, D.L. 1981. Nutrient mass balances for the watershed, headwaters, intertidal zone, and basin of the Rhode River Estuary. Limnology and Oceanography 26: 1142-1149. Correll, D.L., T.E. Jordan, and D.E. Weller. 1992. Nutrient flux in a landscape: effects of coastal land use and terrestrial community mosaic on nutrient transport to coastal waters. Estuaries 15: 431-442. Crenshaw, M. 1980. Mechanisms of shell formation and dissolution, p. 115-128. In D. Rhoads and R. Lutz [eds.], Skeletal growth of aquatic. Plenum Press. Deegan, L.A. and R.H. Garritt. 1997. Evidence for spatial variability in estuarine food webs. Marine Ecology Progress Series 147: 31-47. Dettman, D.L., K.W. Flessa, P.D. Roopnarine, B.R. Schöne, and D.H. Goodwin. 2004. The use of oxygen isotope variation in shells of estuarine mollusks as a quantitative record of seasonal and annual Colorado River discharge. Geochimica et Cosmochimica Acta 68: 1253-1263. Dortsch, Q. 1982. Effect of growth conditions on accumulation of internal nitrate, ammonium, amino acids, and protein in three marine diatoms. Journal of Experimental Marine Biology and Ecology 61: 243-263. Dunton, K.H. 2001. δ15N and δ13C measurements of Antarctic peninsula fauna: Trophic relationships and assimilation of benthic seaweeds. American Zoologist 41: 99-112. Ferguson, J.C. 1982. A comparative study of the net metabolic benefits derived from the uptake and release of free amino acids by marine invertebrates. Biological Bulletin 162: 1-17. France, R.L. 1994. Nitrogen isotopic composition of marine and freshwater invertebrates. Marine Ecology Progress Series 115: 205-207. France, R.L. 1995. Source variability in δ15N of autotrophs as a potential aid in measuring allochthony in freshwaters. Ecography 18: 318-320. Fry, B. 1999. Using stable isotopes to monitor watershed influences on aquatic trophodynamcis. Canadian Journal of Fisheries and Aquatic Sciences 56: 2167-2171. Fry, B. and Y.C. Allen. 2003. Stable isotopes in zebra mussels as bioindicators of riverwatershed linkages. River Research and Applications 19: 683-696. Galindo-Bect, M.S., E.P. Glenn, H.M. Page, K. Fitzsimmons, L.A. Galindo-Bect, J.M. Hernández-Ayon, R.L. Petty, J. Garcia-Hernandez, and D. Moore. 2000. Penaeid shrimp landings in the upper Gulf of California in relation to Colorado River freshwater discharge. Fisheries Bulletin 98: 222-225. Galindo-Bect, M.S., E.P. Glenn, H.M. Page, K. Fitzsimmons, L.A. Galindo-Bect, J.M. Hernández-Ayon, R.L. Petty, J. Garcia-Hernandez, and D. Moore. 2000. Analysis of the Penaeid shrimp catch in the Northern Gulf of California in relation to Colorado River discharge. Fishery Bulletin 98: 222-225. Glibert, P.M., J.C. Goldman, and E.J. Carpenter. 1982. Seasonal variations in the utilization of ammonium and nitrate by phytoplankton in Vineyard Sound, Massachusetts, USA. Marine biology 70: 237-249. Goodfriend, G.A., K.W. Flessa, and P.E. Hare. 1997. Variation in amino acid epimerization rates and amino acid composition among shell layers in the bivalve Chione from the Gulf of California. Geochimica et Cosmochimica Acta 61: 1487-1493. 19 Goodwin, D.H., K.W. Flessa, B.R. Schöne, and D.L. Dettman. 2001. Cross-calibration of daily growth increments, stable isotope variation, and temperature in the Gulf of California bivalve mollusk Chione cortezi: Implications for paleoenvironmental analysis. Palaios 16: 387-398. Goering, J., V. Alexander, and N. Haubenstock. 1990. Seasonal variability of stable carbon and nitrogen isotope ratios of organisms in a North Pacific Bay. Estuarine, Coastal and Shelf Science 30 239-260. Gustafson, L., W. Showers, T. Kwak, J. Levine, and M. Stoskopf. 2007. Temporal and spatial variability in stable isotope compositions of a freshwater mussel: implications for biomonitoring and ecological studies. Oecologia 152: 140-150. Harding, B.L., T.B. Sangoyomi, and E.A. Payton. 1995. Impacts of severe sustained drought on Colorado River water resources. Water Resources Bulletin 31: 815-824. Hastings, J.R. 1964. Climatological data for Baja California. Technical Reports on the Meteorology and Climatology of Arid Regions 18. Hernández-Ayón, J.M., M.S. Galindo-Bect, B.P. Flores-Báez, and S. Alvarez-Borrego. 1993. Nutrient Concentrations are high in the turbid waters of the Colorado River Delta. Estuarine, Coastal and Shelf Science 37: 593-602. Howarth, R.W. 1988. Nutrient limitation of net primary production in marine ecosystems. Annual Review of Ecology & Systematics 19: 89-110. Ingram, B.L., J.C. Ingle, and M.E.Conrad. 1996. A 2000 yr record of Sacramento-San Joaquin river in flow to San Francisco Bay estuary, California. Geology 24: 331-334. Ittekkot, V. and S. Zhang. 1989. Pattern of particulate nitrogen transport in world rivers. Global Biogeochemical Cycles 40: 383-391. Jennings, S. and K.J. Warr. 2003. Environmental correlates of large-scale spatial variation in the δ15N of marine animals. Marine Biology 142: 1131-1140. Jickells, T., J. Andrews, G. Samways, R. Sanders, S. Malcolm, D. Sivyer, R. Parker, D. Nedwell, M. Trimmer, and J. Ridgway. 2000. Nutrient fluxes through the Humber Estuary – past, present, and future. Ambio 29: 130-135. Jorgensen, K.S. 1989. Annual pattern of denitrification and nitrate ammonification in estuarine sediment. Applied and Environmental Microbiology 55: 1841-1847. Kang, C.K., P.-G. Sauriau, P. Richard, and G.F. Blanchard. 1999. Food sources of the infaunal suspension-feeding bivalve Cerastoderma edule in a muddy sandflat of Marennes-Oléron Bay, as determined by analyses of carbon and nitrogen stable isotopes. Marine Ecology Progress Series 187: 147-158. Kaplan, W., I. Valiela, and J.M. Teal. 1979. Denitrification in a salt marsh ecosystem. Limnology and Oceanography 24: 726-734. Kasai, A., H. Horie, and W. Sakamoto. 2004. Selection of food sources by Ruditapes philippinarum and Mactra veneriformis (Bivalvia: Mollusca) determined from stable isotope analysis. Fisheries Science 70: 11-20. Kasai, A. and A. Nakata. 2005. Utilization of terrestrial organic matter by the bivalve Corbicula japonica estimated from stable isotope analysis. Fisheries Science 71: 151-158. Kasai, A., H. Toyohara, A. Nakata, T. Miura, and N. Azuma. 2006. Food sources for the bivalve Corbicula japonica in the foremost fishing lakes estimate from stable isotope analysis. Fisheries Science 72: 105-114. 20 Kemp, W.M., P. Sampou, J. Caffrey, M. Mayer, K. Henriksen, and W.R. Boynton. 1990. Ammonium recycling versus denitrification in Chesapeake Bay sediments. Limnology and Oceanography 35: 1545-1563. Kendall, C. 1998. Tracing nitrogen sources and cycling in catchments, p. 519-576. In C. Kendall and J.J. McDonnell [eds.], Isotope tracers in catchment hydrology. Elsevier. Kiørboe, T. and Mohlenberg, F. 1981. Particle selection in suspension feeding bivalves. Marine Ecology Progress Series 5: 291-296. Kowalewski, M., K.W. Flessa, and J.A.Aggen. 1994. Taphofacies analysis of recent shelly cheniers (beach ridges), northeastern Baja California, Mexico. Facies 31: 209-242. Kowalewski, M., G.A. Goodfriend, and K.W. Flessa. 1998. High-resolution estimates of temporal mixing within shell beds: the evils and virtues of time averaging. Paleobiology 24: 287-304. Kowalewski, M., G.E. Avila-Serrano, K.W. Flessa, and G.A. Goodfriend. 2000. Dead delta’s former productivity: Two trillion shells at the mouth of the Colorado River. Geology 28: 1059-1062. Lake, J.L., R.A. McKinney, F.A. Osterman, R.J. Pruell, J. Kiddon, S.A. Ryba, and A.D. Libby. Stable nitrogen isotopes as indicators of anthropogenic activities in small freshwater systems. Canadian Journal of Fisheries and Aquatic Sciences 58: 870-878. Lavín, M.F. and S. Sánchez. 1999. On how the Colorado River affected the hydrography of the upper Gulf of California. Estuarine, Coastal, and Shelf Science 47: 769-795. L’Helguen, S., C. Madec, and P. Lecorre. 1996. Nitrogen uptake in permanently well-mixed temperate coastal waters. Estuarine, Coastal, and Shelf Science 61: 547-557. Liu, K.-K and I.R. Kaplan. 1989. Eastern tropical Pacific as a source of 15N-enriched nitrate in seawater off southern California. Limnology Oceanography 34: 820–830. Lorrain, A., Y.-M.. Paulet, L. Chauvaud, N. Savoye, A. Donval, and C. Saout. 2002. Differential δ13C and δ15N signatures among scallop tissues: implications for ecology and physiology. Journal of Experimental Marine Biology and Ecology 275: 47-61. Mae, A., T. Yamanaka, and S. Shimoyama. 2007. Stable isotope evidence for identification of chemosynthesis-based fossil bivalves associated with cold-seepages. Palaeogeography, Palaeoclimatology, Palaeoecology 245: 411-420. Mallin, M.A., H.W. Paerl, J. Rudek, and P.W. Bates. 1993. Regulation of estuarine primary production by watershed rainfall and river flow. Marine Ecology Progress Series 93: 199203. Manahan, D.T., S.H. Wright, G.C. Stephens, and M.A. Rice. 1982. Transport of dissolved amino acids by the mussel, Mytilus edulis: Demonstration of net uptake from natural seawater. Science 215: 1253-1255. Mariotti, A., C. Lancelot, and G. Billen. 1984. Natural isotopic composition of nitrogen as a tracer of origin for suspended organic matter in the Scheldt estuary. Geochimica et Cosmochimica Acta 48: 549-555. McClelland, J.W., I. Valiela, and R.H. Michener. 1997. Nitrogen-stable isotope signatures in estuarine food webs: A record of increasing urbanization in coastal watersheds. Limnology and Oceanography 42: 930-937. McKinney, R.A., J.L. Lake, M. Allen, and S. Ryba. 1999. Spatial variability in mussels used to assess base level nitrogen isotope ratio in freshwater ecosystems. Hydrobiologia 412: 1724. 21 McKinney, R.A., J.L. Lake, M.A. Charpentier, and S. Ryba. 2002. Using mussel isotope ratios to assess anthropogenic nitrogen inputs to freshwater ecosystems. Environmental Monitoring and Assessment 74: 167-192. McKinney, R.A., W.G. Nelson, M.A. Charpentier, and C. Wigand. 2001. Ribbed mussel nitrogen isotope signatures reflect nitrogen sources in coastal salt marshes. Ecological Applications 11: 203-214. Millán-Núñez, R., E. Santamaría-del-Ángel, R. Cajal-Medrano, and O.A. Barocio-León. 1999. The Colorado River Delta: A high primary productivity ecosystem. Ciencias Marinas 25: 509-524. Minagawa, M. and E. Wada. 1984. Stepwise enrichment of 15N along food chains: Further evidence and the relation between δ15N and animal age. Geochimica et Cosmochimica Acta 48: 1135-1140. Montoya, J.P., S.G. Horrigan, and J.J. McCarthy. 1990. Natural abundance of 15N in particulate nitrogen and zooplankton in the Chesapeake Bay. Marine Ecology Progress Series 65: 35-61. Needoba, J.A. and P.J. Harrison. 2004. Influence of low light and a light: Dark cycle on NO3uptake, intracellular NO3-, and nitrogen isotope fractionation by marine phytoplankton. Journal of Phycology 40: 505-516. Neff, J.M. 1972. Ultrastructure of the outer epithelium of the mantle in the clam Mercenaria mercenaria in the relation to calcification of the shell. Tissue & Cell 4: 591-600. Nishio, T., I. Koike, and A. Hattori. 1983. Estimates of denitrification and nitrification in coastal and estuarine sediments. Applied Environmental Microbiology 43: 648-653. Nixon, SW. 1980. Between coastal marshes and coastal waters—a review of twenty years of speculation and research on the role of salt marshes in estuarine productivity and water chemistry, p. 437-525. In P. Hamilton and K.B. MacDonald [eds.], Estuarine and wetland processes. Plenum. O’Donnell, T.H., S.A. Macko, J. Chou, K.L. Davis-Hartten, and J.F. Wehmiller. 2003. Analysis of δ13C, δ15N, and δ34 S in organic matter from the biominerals of modern and fossil Mercenaria spp. Organic Geochemistry 34: 165-183. Page, H.M. and M. Lastra. 2003. Diet of intertidal bivalves in the Ría de Arosa (NW Spain): evidence from stable C and N isotope analysis. Marine Biology 143: 519-532. Pegau, W.S., E. Boss, and A. Martínez. 2002. Ocean color observations of eddies during the summer in the Gulf of California. Geophysical Research Letters 29: 9-1 to 9-3. Penschaszadeh, P.E., F. Arrighetti, M. Cledón, J.P. Livore, F. Botto, and O.O. Iribarne. 2006. Bivalve contribution to shallow sandy bottom food web off Mar Del Plata (Argentina): Inference from stomach contents and stable isotope analysis. Journal of Shellfish Research 25: 51-54. Péquignat, E. 1973. A kinetic and autoradiographic study of the direct assimilation of amino acids and glucose by organs of the mussel Mytilus edulis. Marine Biology 19: 227-244. Peterson, B.J., R.W. Howarth, and R.H. Garritt. 1985. Multiple stable isotopes used to trace the flow of organic matter in estuarine food webs. Science 227: 1361-1363. Piola, R.F., S.K. Moore, and I.M. Suthers. 2006. Carbon and nitrogen stable isotope analysis of three types of oyster tissue in an impacted estuary. Estuarine, Coastal and Shelf Science 66: 255-266. 22 Pruell, R.J., B.K. Taplin, J.L. Lake, and S. Jayaraman. 2006. Nitrogen isotope ratios in estuarine biota collected along a nutrient gradient in Narragansett Bay, Rhode Island, USA. Marine Pollution Bulletin 52: 612-620. Raikow, D.F. and S.K. Hamilton. 2001. Bivalve diets in a Midwestern U.S. stream: A stable isotope enrichment study. Limnology and Oceanography 46: 514-522. Rice, M.A. 1999. Uptake of dissolved free amino acids by northern quahogs, Mercenaria mercenaria and its relative importance to organic nitrogen deposition in Narragansett Bay, Rhode Island. Journal of Shellfish Research 18: 547-553. Riera, P., L.J. Stal, and J. Nieuwenhuize. 2000. Heavy δ15N in intertidal benthic algae and invertebrates in the Scheldt estuary (The Netherlands): Effect of river nitrogen inputs. Estuarine, Coastal and Shelf Science 51: 365-372. Riera, P. 1998. δ15N of organic matter sources and benthic invertebrates along an estuarine gradient in Marennes-Oléron Bay (France): Implications for the study of trophic structure. Marine Ecology Progress Series 166: 143-150. Risk, M.J., B.G. Sayer, M.J.S. Tevesz, and C.D. Karr. 1997. Comparison of the organic matrix of fossil and recent bivalve shells. Lethaia 29: 197-202. Rodriguez, C.A., K.W. Flessa, and D.L. Dettman, D.L. 2001a. Effects of upstream diversion of Colorado River water on the estuarine mollusk Mulinia coloradoensis. Conservation Biology 15: 249-258. Rodriguez, C.A., K.W. Flessa, M.A. Tellez-Duarte, D.L. Dettman, and G.A. Avila-Serrano. 2001b. Macrofaunal and isotopic estimates of the former extent of the Colorado River estuary, Upper Gulf of California, Mexico. Journal of Arid Environments 49: 183-193. Rowell, K., K.W. Flessa, D.L. Dettman, and M. Román. 2005. The importance of Colorado River flow to nursery habitats of the Gulf corvina (Cynoscion othonopterus). Canadian Journal of Fisheries and Aquatic Sciences 62: 2874-2885. Santamaría-del- Ángel, E., S. Alvarez-Borrego, and F.E. Müller-Karger. 1994. Gulf of California biogeographic regions based on coastal zone color scanner imagery. Journal of Geophysical Research 99: 7411-7421. Sarakinos, H.C., M.L. Johnson, and M.J. Vander Zanden. 2002. A synthesis of tissuepreservation effects on carbon and nitrogen stable isotope signatures. Canadian Journal of Zoology 80: 381-387. Sato, T., T. Miyajima, H. Ogawa, Y. Umezawa, and I. Koike. 2006. Temporal variability of stable carbon and nitrogen isotopic composition of size-fractionated particulate organic matter in the hypertrophic Sumida River Estuary of Tokyo Bay, Japan. Estuarine, Coastal and Shelf Science 68: 245-258. Schlesinger, W.H. 1997. Biogeochemistry: An analysis of global change, Second edition. Academic Press. Scott, D., J. Harvey, R. Alexander, and G. Schwarz. 2007. Dominance of organic nitrogen from headwater streams to large rivers across the conterminous United States. Global Biogeochemical Cycles 21: GB1003. Sealy, J.C., N.J. Van der Merwe, J.A.L. Thorp, and J.L. Lanham. 1987. Nitrogen isotopic ecology in southern Africa: Implications for environmental and dietary tracing. Geochimica et Cosmochimica Acta 51: 2707-2717. Seitzinger, S.P. and R.W. Sanders. 1997. Contribution of dissolved organic nitrogen from rivers to estuarine eutrophication. Marine Ecology Progress Series 159: 1-12. 23 Seitzinger, S.P. 1988. Denitrification in freshwater and coastal marine ecosystems: Ecological and geochemical significance. Limnology and Oceanography 33: 702-724. Spencer, J.E. and P.A. Pearthree. 2003. Headward erosion versus closed-basin spillover as alternative causes of negogene capture of the ancestral Colorado River by the Gulf of California, p. 215-219. In R.A. Young and E.E. Spencer, E.E. [eds.]. Colorado River: Origin and evolution: Grand Canyon, Arizona. Grand Canyon Association. Sweeney, R.E. and I.R. Kaplan. 1980. Natural abundances of 15N as a source indicator for nearshore marine sedimentary and dissolved nitrogen. Marine Chemistry 9: 81-94. Sweeney, R.E., K.-K. Liu, and I.R. Kaplan. 1978. Organic nitrogen isotopes and their uses in determining the source of sedimentary nitrogen, p. 9-26. In B.W. Robinson [ed.], Stable isotopes in the earth science. DSIR Bull. 220, New Zealand Dept. Scientific and Industrial. Thompson, D.A. 1999. Tide calendar for the Northern Gulf of California. Arizona, USA. Printing and Graphic Services, University of Arizona. Thompson, R.W. 1968. Tidal flat sedimentation on the Colorado River delta, northwestern Gulf of California. Memoir 107. Geological Society of America. Thurman, E.M. 1985. Organic geochemistry of natural waters. Martinus Nijhoff/Dr. W. Junk Publishers. Vallentyne, J.R. 1969. Pyrolysis of amino acids in Pleistocene Mercenaria shells. Geochimica et Cosmochimica Acta 33: 1453-1458. Valiela, I. and J.M. Teal. 1979. The nitrogen budget of a salt marsh ecosystem. Nature 280: 652656. Wada, E., M. Minagawa, H. Mizutani, T. Tsuji, R. Imaizumi, and K. Karasawa. 1987. Biogeochemical studies on the transport of organic matter along the Otsuchi River watershed, Japan. Estuarine Coastal, and Shelf Science 25: 321-336. Waser, N.A., Z. Yu, K.Yin, B. Nielsen, P.J. Harrison, D.H. Turpin, and S.E. Calvert. 1999. Nitrogen isotopic fractionation during a simulated diatom spring bloom: importance of N-starvation in controlling fractionation. Marine Ecology Progress Series 179: 291-296. Waser, N.A., K. Yin, Z. Yu, K. Tada, P.J. Harrison, D.H. Turpin, and S.E. Calvert, S.E. 1998. Nitrogen isotope fractionation during nitrate, ammonium and urea uptake by marine diatoms and coccolithophores under various conditions of N availability. Marine Ecology Progress Series 169: 29-41. Weinter, S. and L. Hood. 1975. Soluble protein of the organic matrix of mollusk shells: A potential template for shell formation. Science 190: 987-989. Wilbur, K.M. 1964. Shell formation and regeneration, p.243-282. In K.M. Wilbur and C.M. Yonge [eds.], Physiology of Mollusca, Vol. 1. Acadmic Press. Yelenik, S., J. McClelland, N. Feinstein, and I. Valiela. 1996. Changes in N and C stable isotope signatures of particulate organic matter and ribbed mussels in estuaries subject to differential nutrient loading. Biology Bulletin 191: 329-330. Yokoyama, H., A. Tamaki, K. Harada, K. Shimoda, K. Koyama, and Y. Ishihi. 2005. Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Marine Ecology Progress Series 296: 115-128. York, J.K., G. Tomasky, I. Valiela, and D.J. Repeta. 2007. Stable isotopic detection of ammonium and nitrate assimilation by phytoplankton in the Waquiot Bay estuarine system. Limnology and Oceanography 52: 144-155. 24 Table 1. δ15N values reported for suspension-feeding bivalve mollusks in various environments LOCATION ENVIRONMENT SPECIES TISSUE low Coringa Wildlife Sanctuary and Kakinada Bay, Andra Pradesh, India San Francisco Bay, USA Mangrove creeks and estuary Creek Creek Creek Creek Bay Bay Bay Bay Bay Bay Bay Salt marsh Meretrix meretrix Tellina spp. Macoma spp. Placuna placenta All bivalves - creek Meretrix meretrix Tellina spp. Macoma spp. Placuna placenta Paphia undulata Pinctada radiata Siliqua albida All bivalves - bay Stomach content Stomach content Stomach content Stomach content Macoma petalum Whole body Stomach content Stomach content Stomach content Stomach content Stomach content Stomach content Stomach content 8.1 6.2 8.5 8.7 6.2 9.5 6.8 8.9 9.3 7.7 11.6 7.6 7.6 δ15N high mean 8.1 8.0 8.5 9.1 9.1 12.0 9.3 8.9 9.5 12.9 11.6 7.6 12.9 8.1 7.2 8.5 8.9 7.6 9.9 7.7 8.9 9.4 9.7 11.6 7.6 9.0 14-15 Comment Source Limited utilization of mangrove and terrestrial sources. Local algal sources of OM are dominant portion of diet. δ15N of tissues from bivalves in creek < Bouillon et al. (2002) bay; ~3.2 permil gradient between the environments for bivalves and other benthic invertebrates No significant differences in δ15N between salt marsh habitats Brusati and Grosholtz (2007) Estuary Upper ARE Rangea cuneata (93%) Middle ARE and Corbicula fluminea Lower ARE (7%) All bivalves - ARE Upper NRE Rangea cuneata (93%) Middle NRE and Corbicula fluminea Lower NRE (7%) All bivalves - NRE Neuse River Estuary (NRE) and Alligator River Estuary (ARE), North Carolina, USA Various Various Lampsilis sp., Anodonta Ontario and Quebec, Freshwater lakes Canada sp., Elliptio spp. Lake Oneida, USA Freshwater lakes Dreissena polymorpha Assiminea lutea, Gamo Lagoon, Nuttalia olivacea, Nanakita River Estuary Corbicula japonica, Estuary, Japan Laternula limicola Estuary Pleasant Bay, Cape Multiple subestuaries Mercenaria mercenaria Cod, USA Estuary Modiolus demissus Plum Island Sound, Middle estuary Crassostrea virginica Massachusetts, USA Mya arenia Lower estuary Mya arenia Antarctic Peninsula, near Anvers Island Laternula elliptica Nearshore marine Yoldia eightsi Foot Foot Foot 7.4 6.3 6.1 6.4 10.7 10.9 9.3 10.4 Foot Foot Foot ? 1.2 9 ? ~8 ? ~9.5 Whole body Muscle Muscle Muscle Muscle Muscle or "body wall tissue" Muscle or "body wall tissue" 8 High variability. δ15N values correlate Cabana and with degree of anthropogenic N loading Rasmussen (1996) and human population within watershed δ15N values correlate with % wastewater ~6.5 ~10.5 7.1 δ15N values of bivalves from NRE > ARE; POM follows same trend. Reflects higher wastewater and animal waste N Bucci et al. (2007) in Neuse. δ15N decline toward sea due to high-δ15N N loading upstream 7.8 6.5 8.3 8 Carmichael et al. (2004) Deegan and Garritt 5.7 Dunton (2001) 6.5 Estuary North Bay San Francisco Bay, USA South Bay - spring bloom 1990 South Bay - spring bloom 1991 Potamorcorbula amurensis Potamorcorbula amurensis Potamorcorbula amurensis Whole (minus gut contents) Whole (minus gut contents) Whole (minus gut contents) 10.7 δ15N values elevated in South Bay 11.5-11.9 12 relative to North Bay due to stronger Fry (1999) 14.3 anthropogenic N loading. Values within North Bay higher upstream due to 13 anthropogenic inputs River Baton Rouge Dreissena polymorpha Illinois Dreissena polymorpha Mississippi River, USA Whole body Whole body 11 10 16 16 Auke Bay, Alaska, USA Macoma nasuta Saxidomus giganteus Yoldia spp. Whole body Whole body Whole body 6.1 8.6 Estuary Piedmont region, North Carolina, USA Freshwater streams Elliptio complanata Foot 4.9 9.1 Northeast Atlantic Ocean (Irish Sea, English Channel, North Sea) Offshore marine Aequipecten opercularis Adductor muscle 4.18 11 7.6 9.3 8.5 11.6 10.3 11.5 Marennes-Oléron Bay, Estuary France Certastoderma edule Whole body Estuary Kushida Estuary, Japan Japan Upper estuary Corbicula japonica Lower estuary Corbicula japonica Coastal brackish lakes Lake Jusan Upstream Downstream Lake Ogawara Upstream Downstream Lake Shinji Upstream Downstream Miya Estuary, Ise Bay, Estuary Japan Rhode Island, USA Bay of Brest, France Freshwater lakes and ponds Marine Corbicula japonica Corbicula japonica Corbicula japonica Corbicula japonica Corbicula japonica Corbicula japonica Corbicula japonica Corbicula japonica Corbicula japonica All bivalves Foot Foot Foot Foot Foot Foot Foot Foot Foot Foot Foot Ruditapes philippinarum Muscle Muscle Mactra veneriformis Elliptio complanata ? Pecten maximus Pecten maximus Adductor muscle Gonad 11.7 10.2 8.5 11.7 9.9 10.2 10.6 11.2 4.9 13.3 Seasonal changes as large as 5 ‰ (in Baton Rouge); max values at end of summer phytoplankton bloom and low in early spring; spatial differences due to anthropogenic loading Fry and Allen (2003) 7.6 Significant seasonal differences in δ15N Goering et al. (1990) 9.1 of Macoma 7.6 Bivalve δ15N correlate with long-term 7.5 Gustafson et al. (2007) means of dissolved nitrate δ15N Jennings and Warr (2003) 7.45 Relative importance of 8.4 microphytoplankton vs. algal POM Kang et al. (1999) inferred from δ15N Enriched values in lower estuary reflect Kasai and Nakata 8.6 greater % marine POM; depleted 11.8 values in upper estuary reflect greater (2005) % terrestrial POM 10.4 9 11 11 11.1 10.9 10.9 10.6 11.4 10.8 Gradient in δ15N between river mouth and downstream areas in Lake Jusan (increasing toward ocean); less apparent in other lakes Kasai et al. (2006) High % terrestrial POM but little Kasai et al. (2004) 10.1 utilization by the bivalves 10.5 Elevated δ15N correlate with a higher % 8.4 residential development & wastewater Lake et al. (2001) N 9.4 7.8 Significant separation in δ15N of Lorrain et al. (2002) Bay of Brest, France Marine Pecten maximus Pecten maximus Digestive gland Whole body (est.) Estuary Childs River Estuary Sage Lot Pond Estuary Mya arenia Mya arenia Whole body Whole body Narragansett Bay, Rhode Island, USA 22 Salt marshes Geukensia demissa Whole body Rhode Island 19 Freshwater lakes Elliptio spp. Waquoit Bay, Cape Cod, Massachusetts, USA Rhode Island Usujiri coast, Hokkaido, Japan Freshwater ponds, lakes, reservoirs Quidnick Reservoir Elliptio spp. Elliptio spp. Elliptio spp. Elliptio spp. Tucker Pond Elliptio spp. Elliptio spp. Elliptio spp. Elliptio spp. Browning Mill Pond Elliptio spp. Elliptio spp. Elliptio spp. Elliptio spp. Spring Lake Elliptio spp. Elliptio spp. Elliptio spp. Elliptio spp. Mishnock Lake Elliptio spp. Elliptio spp. Elliptio spp. Elliptio spp. Tiogue Lake Elliptio spp. Elliptio spp. Elliptio spp. Elliptio spp. All bodies Elliptio spp. Elliptio spp. Elliptio spp. Septifer virgatus Marine Mytilus edulis Marine Assateague Channel, Mercenaria mercenaria Chincoteague, VA Mercenaria mercenaria Mercenaria mercenaria Mercenaria mercenaria Mercenaria mercenaria Mercenaria mercenaria Mercenaria Cedar Key, FL campechiensis Gomez Pit, Virginia Mercenaria mercenaria Beach, VA Atlantic coastal plain Mercenaria mercenaria Mercenaria campechiensis Bass site, North Carolina Mercenaria mercenaria shelf Mark Clark Pit, Mercenaria mercenaria Charleston, SC Jones site, Skidaway Mercenaria mercenaria Island, Georgia Ria de Arosa, Spain Estuary Northern Argentina Coastal marine Great Sippewissett Marsh, Falmouth, Massachusetts, USA Salt marsh Manning River Estuary, New South Wales, Australia Estuary All sites Mercenaria mercenaria Cerastoderma edule Cerastoderma edule Tapes decussatus Tapes decussatus Mytilus galloprovincialis Mytilus galloprovincialis Amiantis purpurata Solen tehuelchus 12.1 Foot tissue 4.9 12.6 Adductor muscle Foot tissue Mantle tissue Mean of 3 tissues Adductor muscle Foot tissue Mantle tissue Mean of 3 tissues Adductor muscle Foot tissue Mantle tissue Mean of 3 tissues Adductor muscle Foot tissue Mantle tissue Mean of 3 tissues Adductor muscle Foot tissue Mantle tissue Mean of 3 tissues Adductor muscle Foot tissue Mantle tissue Mean of 3 tissues All muscle All foot All mantle Whole body Whole body 6.2 4.7 4.3 6.5 5.1 4.8 6 5.1 5 6.5 5.2 5.3 6.9 5.9 6 7.2 6.7 6.3 8.8 8.2 8.2 8.9 8.3 9 9.9 8.6 8.3 10.6 9.6 9.3 13.1 11.7 11.2 13.4 11.9 11.6 Modern shell (Inner & middle layers, ventral margin) Modern shell (ventral margin) Foot Mantle Muscle All soft tissue Modern shell (middle layer) Fossil shell (Late Pleistocene; inner & middle layers, ventral margin) Fossil shell (Mid Pleistocene; inner & middle layers, ventral margin) Fossil shell (Early Pleistocene; inner & middle layers, ventral margin) Fossil shell (Holocene; inner & middle layers, ventral margin) Fossil shell (Late Pleistocene; middle layer) Fossil shell (Late Pleistocene; middle layer) All shells Stomach Muscle Stomach Muscle Stomach Muscle Stomach content Stomach content Geukensia demissa Muscle Saccostrea glomerata Saccostrea glomerata Adductor muscle Ctenidia Viscera (= whole body ctenidia & adductor muscle) Whole body Saccostrea glomerata Saccostrea glomerata Estuary 9 11 13.7 10.1 11.6 7.2 9.9 4.5 13.2 7.2 9.3 6.5 9.3 3.7 9.8 3.8 4.5 7.2 3.7 7.2 13.7 Significant separation in δ N of 6 different tissues 8.5 Bivalves in Childs River Estuary ~7.9 enriched relative to Sage Lot Pond ~5.6 Estuary due to stronger wastewater influence Bivalves with diets dominated by terrestrial and marsh sources show depleted δ15N; where marine sources dominate = enriched δ15N. Values correlate with land use practices Correlation with % residential development and often with dissolved nitrate concentration 6.4 4.9 4.6 5.3 6.3 5.2 5.1 5.5 7.1 6.3 6.2 6.5 8.8 8.2 8.5 8.5 10.3 9.2 8.9 9.5 13.2 11.9 11.4 12.2 8.7 7.6 7.5 9 8.7 ~8.5 8.5 6.9 10.4 10.1 4.6 2.9 8.7 10.1 McClelland et al. (1997) McKinney et al. (2001) McKinney et al. (2002) Adductor muscle consistently enriched relative to other tissues. All tissues enriched in locations with stronger anthropogenic N loading McKinney et al. (1999) δ15N independent of age and nitrogen mass balance in bivalve body Minagawa and Wada (1984) Very large intrashell variation. Mean separation between tissue and shell material at the ventral margin = 0.7 ‰ O'Donnel et al (2003) 10.6 11.5 11 12.2 11.3 9.5 10.7 10.1 11.3 9.6 10.8 12.3 10.9 ~5.5 Lorrain et al. (2002) Muscle tissue enriched relative to stomach tissue. Spatial pattern in δ15N values from multiple stations does not Page and Lastra (2003) suggest utilization of riverine N (freshwater inputs small relative to tidal exchange) Values suggestive of low trophic Penschaszadeh (2006) position Generally lower δ15N values for individuals from innermost marsh, increasing toward the ocean Peterson et al. (1985) Bivalves transplanted from Wallis Lake, New South Wales. Measured after 3 mo. δ15N records position in estuary Piola et al. (2006) (enriched seaward) and sewage outfall. Suggests seasonal information can be 6.8 recorded in tissue. Passeonkquis Cove (Upper Bay) Geukensia demissa Prudence Island (Middle Bay) Geukensia demissa Fox Hill Salt Marsh (Lower Bay) Geukensia demissa Sphaerium striatinum Pleurobema sintoxia + Eagle Creek, Freshwater creek Fusconaia flava Michigan, USA Pleurobema sintoxia + Fusconaia flava Estuary Oosterschelde Estuary Cerastoderma edule Oosterschelde Estuary Crassostrea gigas Scheldt Estuary, Oosterschelde Estuary Mytilus edulis Netherlands Westerschelde Estuary Cerastoderma edule Westerschelde Estuary Crassostrea gigas Westerschelde Estuary Mytilus edulis Estuary Port Neuf (freshwater end) Crassostrea gigas Marennes-Oléron Bay, Fort Lupin Crassostrea gigas France Les Palles Crassostrea gigas Chassiron (marine end) Crassostrea gigas Les Baleines (marine end) Crassostrea gigas Corbicula fluminea Unknown Freshwater? Corbicula fluminea Naragansett Bay, Rhode Island, USA Elands Bay, SW Cape Marine of South Africa Estuary Waquoit Bay, Sage Lot Pond Estuary Massachusetts, USA Quashnet River Estuary Childs River Estuary Mouth of Shirakawa River, Ariake Sound, Western Kyushu, Japan Estuary Whole body 10.4 11.6 Whole body 10.7 11.2 Whole body Whole body 10.0 10.2 10.1 4.4 Muscle 4.8 Stomach 3.3 Whole body Whole body Whole body Whole body Whole body Whole body 10.4 10.1 10.9 18.7 19.6 ~18 Whole body Whole body Whole body Whole body 11.5 10 9 8.3 Whole body Whole body? Whole body? Chromytilus meridionalis ? Geukensia demissa Geukensia demissa Geukensia demissa Mactra veneriformis (juvenile) Ruditapes philippinarum (juvenile) Low intra-annual and minor interannual variability. Significant spatial trend Pruell et al. (2006) (depleted oceanward) that likely relates 10.9 to sewage inputs 11.0 Dominance of deposit feeding (vs. suspension feeding) inferred Raikow and Hamilton (2001) Elevated tissue δ15N in Westerschelde Estuary, which is more affected by urbanization. Utilization of Riera et al. (2000) riverine/terrestrial material is low while utilization of 15N-enriched (from wastewater) benthic algae is high POM more enriched seaward, yet oysters show opposite trend. Suggests that oysters at head of estuary at higher Riera (1998) trophic level (not feeding directly on terrestrial detritus but perhaps on bacterial intermediary). 8.8 6.7 Tissue preservation (formalin, EtOH) on Sarakinos et al. (2002) 6.3 archived specimens found to not produce δ15N shift > 0.5 ‰ 8.1 8.9 8.46 Sealy et al. (1987) 15 Whole body Whole body Whole body Both bivalve and POM δ N track the 7.4 level of anthropogenic N 8.4 (Childs>Quashnet>Sage) 9.3 Whole body 8.2 Whole body 8.5 Yelenik et al. (1996) Yokoyama et al. (2005) Las Isletas Fig. 1. Study area. (a) Regional map of Baja, Mexico and Gulf of California. (b) Colorado River Delta and Upper Gulf of California, with bivalve collection sites identified by arrows. Adapted from Rodriguez et al. (2001a). Ground & homogenized for whole-shell δ15N average (Umbo) Summer growth bands Ventral margin (most recent growth) Fig. 2. Sampling scheme for whole-shell, margin, and summer shell powder Spot samples for δ18O measurement Table 2. Rooftop conditions during diagenesis experiment. Max temp Min temp Avg temp Max light (oC) (oC) (oC) Month (Lux) April (19th-) 58 7 28 231468 May 65 10 34 231468 June 67 15 39 220446 July 67 20 37 209424 August 63 21 37 165334 Sept (-29th) 62 15 34 159823 Table 3. Mean whole-shell δ15N of Corbicula collected from the Lower Colorado R. drainage Collection site Mitry Lake Imperial Lake Island Lake Lake Mead Yuma, AZ Walter's Camp Mean Location ID A F L M Y W δ15N (‰) 4.88 10.93 9.88 12.51 9.60 9.52 9.55 Table 4. All δ15N values obtained from the shells of bivalves collected from the Colorado River estuary and the lower Colorado River drainage basin Lower Colorado River Lower Colorado River Coribcula fluminea Unionid mussels 15 Shell ID Shell area δ N (‰) Shell ID Shell area δ15N (‰) margin 2.73, 3.43 S1 whole 5.77 A1 whole 4.81 U1 whole 5.59 margin 5.18 A2 whole 3.99 margin 3.15 A3 whole 4.81 margin 2.12 A4 whole 4.58 AX1-control whole 4.96 AX2-control whole 6.11 FX1-control whole 10.90 FX2-control whole 10.97 L1 whole 9.03 L2 whole 10.73 W1 whole 9.83 W2 whole 9.22 Y1 whole 9.48 Y2 whole 9.72 M1 whole 11.18 M2 whole 13.50 M3 whole 12.58 12.11a whole M4 13.18b whole MEAN MEAN§ MEAN$ margin whole whole 3.38 MEAN 8.61 9.55 a = Value from buried shell valve b = Value from exposed shell valve * = Value based on cross-sectional slice § = Based on all whole-shell values $ = Based on location means (A, F, L, P, W, Y, M) whole Colorado River Delta Chione fluctifraga (modern) Shell ID Shell area δ15N (‰) margin 9.48 B1 whole 14.91* B2 whole 14.26* margin 9.39, 10.37 B3 whole 15.03* margin 5.96 B4 whole 14.47* summer 13.52 BX1-control whole 15.43* BX2-control whole 14.43 BX3-control whole 15.08* 5.68 MEAN MEAN MEAN margin whole summer Colorado River Delta Chione fluctifraga (pre-dam) Shell ID Shell area δ15N (‰) whole 12.41 G1 early summer 11.87 whole 15.39 G2 early summer 15.59 whole 12.84 G3 early summer 12.26 G4 whole 14.90 G5 whole 14.72 G6 whole 16.00 G7 whole 16.10 8.44 MEAN 14.77 MEAN 13.52 whole early summer Colorado River Delta Mulinia coloradoensis (modern) Shell ID Shell area δ15N (‰) margin 11.46 C1 whole 11.49 margin 11.84 C2 whole 12.32 margin 9.08, 10.94 C3 whole 13.40 margin 13.28 C4 whole 13.56 summer 13.22 CX1-control whole 14.36 CX2-control whole 13.31 CX3-control whole 14.71 14.62 MEAN 13.24 MEAN MEAN margin whole summer Colorado River Delta Mulinia coloradoensis (pre-dam) Shell ID Shell area δ15N (‰) D1 whole 14.11 D5 whole 14.31 D6 whole 13.08 D7 whole 12.20 D8 whole 13.07 D9 whole 12.82 whole 12.95 D10 early summer 8.76 7.74*, whole D11 7.89*, 9.63 early summer 9.04 whole 7.96* D12 early summer 9.21, 9.71 11.58 MEAN 13.87 MEAN 13.22 whole early summer 12.14 9.09 Table 5. δ18O values obtained from shell carbonate. Gray = shell growth during the early summer flood pulse (predam); Yellow = summer shell growth (modern) Pre-dam Mulinia Drill sample 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 Early summer 1 (predicted) Early summer 1 (sampled) Early summer 2 (predicted) Early summer 2 (sampled) Shell D10 Shell D11 Shell D12 δ18O δ18O δ18O -1.68 -1.68 -3.90 -3.11 -3.65 -4.19 -3.63 -4.44 -4.55 -3.49 -4.96 -3.79 -3.23 -4.30 -1.29 -3.50 -4.20 -1.39 -3.74 -2.84 -1.19 -3.42 -3.91 -2.33 -3.92 -1.88 -3.28 -4.67 -1.91 -3.55 -4.27 -1.90 -5.38 -4.79 -1.70 -5.02 -4.53 -2.45 -3.16 -3.43 -2.28 no data -3.62 -2.91 -3.26 -3.12 -3.39 -3.29 -2.39 -3.04 -2.38 -2.02 -4.08 -2.50 -2.37 -1.00 -2.56 -0.76 -4.15 -4.13 -4.31 -4.73 -4.11 -4.13 -4.08 -4.28 Modern Modern Pre-dam Chione Mulinia Chione Shell C4 Shell G1 Shell G2 Shell G3 Shell B4 δ18O δ18O δ18O δ18O δ18O -1.43 0.03 0.48 0.86 -0.71 -1.24 0.13 0.05 0.56 -1.38 -1.56 -0.34 0.43 0.24 -1.52 -1.65 -0.43 0.61 0.56 -1.72 -0.37 -0.70 0.65 0.48 -1.68 1.89 -2.28 no data 0.04 -2.14 1.21 -2.72 no data -0.05 -2.24 1.74 -3.43 0.78 0.18 -2.15 1.69 -2.89 0.35 -0.65 -2.30 1.19 -4.78 0.30 -2.23 -2.47 0.87 -5.18 0.23 -3.18 -2.16 0.30 -3.18 -0.75 -3.03 -0.41 -1.58 -3.43 -4.44 -1.08 -1.41 -2.01 -4.16 -1.64 -1.09 0.83 -2.94 -0.70 -0.75 -2.32 -0.20 -2.24 -0.78 0.12 -3.43 0.44 -1.19 -2.60 0.68 -1.29 -2.53 0.70 -1.43 -4.86 -5.32 -3.79 -1.88 -1.40 -1.47 -4.01 -2.70 -3.19 -2.26 -1.64 -3.62 -2.85 -3.53 -2.10 -4.66 NS Mulinia Shell D10 2.00 Shell G1 2.00 1.00 1.00 0.00 0.00 -1.00 -1.00 -2.00 -2.00 -3.00 -3.00 -4.00 -4.00 -5.00 -5.00 -6.00 -6.00 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 Shell D11 2.00 0 2.00 1.00 2 4 6 8 10 12 14 16 18 20 22 24 26 28 8 10 12 14 16 18 20 22 24 26 28 8 10 12 14 16 18 20 22 24 26 28 8 10 12 14 16 18 20 22 24 26 28 Shell G2 1.00 0.00 0.00 -1.00 -1.00 -2.00 -2.00 -3.00 -3.00 -4.00 -4.00 -5.00 -5.00 -6.00 Pre-dam Fig. 3. δ18O values (y-axis) obtained from shell carbonate. Sample points (xaxis) were taken by drill at the outer shell surface along a short transect between the umbo and ventral margin. Boxed areas represent summertime shell deposition and were subsequently sampled for δ15N measurement. Chione -6.00 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 Shell D12 2.00 0 2.00 1.00 2 4 6 Shell G3 1.00 0.00 0.00 -1.00 -1.00 -2.00 -2.00 -3.00 -3.00 -4.00 -4.00 -5.00 -5.00 -6.00 -6.00 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 0 Shell C4 2.00 2 4 6 Shell B4 2.00 1.00 1.00 0.00 -1.00 -1.00 -2.00 -2.00 -3.00 -3.00 -4.00 -4.00 -5.00 -5.00 Modern 0.00 -6.00 -6.00 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 0 2 4 6 Table 6. Differences between whole-shell and summer-shell δ15N values Species Pre-dam shells D10 D11 D12 G1 G2 G3 Modern shells C4 B4 M. coloradoensis Whole-shell Summer-shell δ15N δ15N M. coloradoensis Ch. fluctifraga Ch. fluctifraga Ch. fluctifraga 12.95 7.74, 7.89, 9.63 7.96 12.41 15.39 12.84 M. coloradoensis Ch. fluctifraga 13.56 14.47 M. coloradoensis Difference 8.76 4.19 9.04 -1.30 to 0.59 9.21, 9.71 11.87 15.59 12.26 -1.25 to -1.75 0.54 -0.20 0.58 13.22 13.52 0.33 0.95 Table 7. δ15N values obtained in diagenesis experiment. Shell δ15N (‰) Shell ID A1X A2X F1X F2X C1X C2X C3X B1X B2X B3X Control 4.96 6.11 10.90 10.97 14.36 13.31 14.71 15.23 14.43 15.08 Roof 3.48 6.71 10.68 10.87 14.60 13.91 14.38 14.26 14.79 14.60 Oven 3.93 5.44 10.29 10.50 14.46 12.64 13.66 14.78 14.03 13.49 Roof δ15N shift (‰) Oven δ15N shift (‰) Difference Difference Direction Direction (control(controlof change of change roof) oven) -1.48 -1.03 0.60 + -0.67 -0.22 -0.61 -0.10 -0.47 0.24 + 0.10 + 0.60 + -0.67 -0.33 -1.05 -0.97 -0.45 0.36 + -0.40 -0.48 -1.59 - 1.00 Oven-control 0.50 0.00 δ15N (‰) - -0.50 -1.00 -1.50 -2.00 Fig. 4. Diagenesis experiment: Difference in δ15N between control and experimental groups Roof-control Table 8. Turnover rates for bivalve tissues. Species Freshwater stream Foot Stomach Gut contents Turnover time Source (days) 357 Raikow and Hamilton 78 (2001) 85 Whitsand Bay, Southwest England Marine Whole body 250-345 Hawkins (1985) North Carolina Freshwater streams Hemolymph 113 Gustafson et al. (2007) Naragansett Bay, Rhode Island Salt marshes Collection location 12 species of Unionid Eagle Creek, mussels Southern Michigan Mytilus edulis Elliptio complanata Geukensia demissa Environment Tissue Whole body (30 mm animal) Whole body (50 mm animal) 206 McKinney et al. (2001) 397