n ti Point of Use (POU) Gas Purification for
advertisement

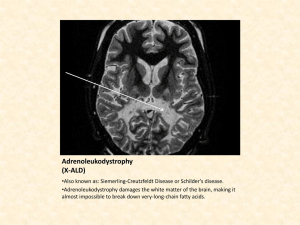
Point of Use (POU) Gas Purification for Atomic Layer Deposition (ALD) Processes Anthony Ricci ALD Process Overview A promising new method of depositing very thin films has been developed for a variety of applications, including gate dielectrics, capacitor dielectrics, and diffusion barriers.1 The new technique, called atomic layer deposition (ALD), solves many of the most significant problems associated with traditional thin-film deposition technologies, including conformal film deposition over high-aspect ratio structures, high-temperature processing, large-area film uniformity, and accurate film thickness control. Traditional thin-film deposition technologies, such as physical vapor deposition (PVD) require ultra-high vacuum conditions and highdensity plasmas for accurate metal or metal nitride deposition over high-aspect ratio structures, and are often plagued by conformality and plasma damage problems. With chemical vapor deposition (CVD) processes, accurate film thickness and uniformity over large areas are difficult to control. ALD uses a precursor and a reactant, which are introduced separately in a pulsed fashion to bond chemically with the heated wafer surface until self-limiting surface saturation is reached (all available surface sites are occupied). The precursor and reactant pulses are performed with fast -actuating valves in order to control the amount of precursors introduced into the reactor chamber. An inert purge gas separates the pulses, carrying away unreacted precursor and reactant molecules, and surface reaction byproduct molecules. ALD reactions will only take place if the starting surface is prepared to chemically react directly with each precursor and reactant type. The sequence of surface reactions (precursor + inert gas purge + reactant + inert gas purge) is called the ALD deposition cycle (Figure 1).2 Figure 1. Generic ALD Process Flow Time Flow, Pressure Application Bulletin ABG-105-0305 Cycle time A B A B A B A B A B A B A B Process Parameters: • Reaction A • A Purge • Rx B (Plasma Opt) • B Purge Courtesy of Genus Figure 2. Contaminants in ALD Processes • heated walls are recommended to reduce physisorption of undesirable molecules, • diffusion of precursors limits control of pulse separation, and • purge gas velocity governs the macro flow. Deposition of an ALD Al2O3 Layer Ti (first layer) ALD Chemical Framework The precursors and reactants used in ALD are introduced into the ALD reactor separately. This is done by separating precursors and reactants with an inert gas (N2 or Ar) purge step. Ti (second layer) Dimethylamino or Diethylamino N Top surface layer in <111> plane of TiN Adsorption of Ti(NR2)4 on Ti surface TiN. Coverage is limited due to steric hinderance between adsorbed precursor molecules because of interference of ligands on adjacent precursor molecules After exposure to NH3, the ligands are removed leaving a partial monolayer coverage of Ti and N. Subsequent exposure will then fill in the gaps. Courtesy of Novellus The surface-saturated film growth process in ALD ensures excellent conformality and step coverage, excellent within-wafer and waferto-wafer uniformity, and accurate film thickness control. All of these aspects are critical when depositing ultra-thin films on high-aspect ratio structures. ALD Tools The ALD reactor can be either single or batch; the following are key reactor characteristics2: • self-limiting growth ensures precursor fluxes do not need to be uniform over the wafer, • rapid precursor switching and purging is needed, 2 For example, in the deposition of tantalum nitride films, first hydrogen gas is passed over a SiN layer, forming SiN-H to promote tantalum adhesion. Then the tantalum metal precursor pulse reacts with the SiN-H layer to form SiNTaCl3 and HCl vapor. An inert purge gas step is used to remove the HCl vapor from the ALD reactor. To continue from the previous example, the nitride layer is deposited on the tantalum layer by pulsing the reactant gas (NH3) to form SiNTa-N. Again, HCl is the volatile byproduct molecule. An inert purge gas step removes the reaction byproduct (HCl) and any unreacted reactant (NH3) from the vacuum chamber. A sub-nm conformal layer of TaN has been deposited. One typical cycle deposits roughly 1 Å of film in a few seconds. The final film thickness may be precisely determined by controlling the number of deposition cycles (Figure 2).3 Contaminants in ALD Processes The purity of the purge, carrier, and reactant gases, and the control of outgassed materials that were physisorbed to the reactor walls, are key parameters because ALD is a relatively slow and low-temperature process, compared to other deposition technologies.3 As a result, molecular contamination has a greater negative effect on film quality and throughput. Also, in order to reduce operating temperatures (lowtemperature ALD), longer purge gas steps are needed to achieve highly uniform films.4 Highpurity inert gases such as nitrogen or argon can contain combined water and oxygen contamination levels as much as 2 ppm.5 These inert purge gases are used to remove the volatile byproduct and any unreacted precursor and reactant molecules. Molecular contamination in carrier gases such as hydrogen could disintegrate precursor species and be deposited onto the reactor walls. POU Purification for ALD Processing The placement of purifier assemblies in the purge, carrier, and ammonia reactant gas lines directly prior to reactor entry (i.e., at the pointof-use) will provide the best defense against contamination from entering the reactor. Pall AresKleen™ purification medium is capable of removing gaseous contamination such as water, oxygen, carbon monoxide, and carbon dioxide to sub-ppb levels from inert purge and carrier gases, such as nitrogen, argon, and hydrogen. Pall Gaskleen® purifier assemblies are available in Top Mount and in-line configurations. (Refer to Pall Gas Filtration Purifiers Datasheets.) POU filtration is effective at removing particulate contamination ≥ 0.4 microns at 99.9% removal efficiency in the low-vapor pressure precursors by using the in-line Pall Gasket-Sert™ PSP filter. (Refer to Pall Gas Filtration Metal Datasheet.) Glossary Aspect ratio – the ratio of height-to-width of a given step, trench, or contact hole. Chemisorption – to chemically (irreversibly) bind a substance on the surface of another substance. Conformality – a measure of how well a deposited layer conforms to the layer beneath it. References 1. Markku Leskela, “ALE research 6/99 – 9/00,” University of Helsinki, Laboratory of Inorganic Chemistry. 2. Tom Seidel, “ALD Reactor Architectures,” NSFSRC Network Forum, (4/24/2003). 3. Aaron Hand, “Industry Begins to Embrace ALD,” Semiconductor International, (5/1/2003). 4. M.J. Biercuk, D.J. Monsma, C.M. Marcus, J.S. Becker, and R.G. Gordon, Applied Physics Letters 83, 12 (2003). 5. Nitrogen, Matheson Purity 99.9995%, www.mathesontrigas.com. Gas Filtration Purifiers Data Sheet A79a Mini-Gaskleen™ Purifier Gas Filtration Purifiers Data Sheet A88 Gaskleen® II Purifier Gas Filtration Purifiers Data Sheet A87a Gaskleen® ST Purifier Gas Filtration Purifiers Data Sheet A81a Maxi-Gaskleen™ Purifier Gas Filtration Purifiers Data Sheet A86a Gaskleen® 11⁄8” C-seal Purifier Gas Filtration Metal Data Sheet A94 Gasket-Sert™ PSP Filter Deposition rate – the rate at which a given film is deposited. It is usually measured in Angstroms/minute. Physisorption – to physically (reversibly) bind a substance on the surface of another substance. Step coverage – the degree to which a film can evenly cover the sides and bottom of vertical features, such as trenches and contact openings. Uniformity – a measure of how far a given parameter, such as deposition rate, varies from the average value across one wafer, from wafer to wafer in a batch, or from wafer batch to batch. 2200 Northern Boulevard East Hills, New York 11548-1289 USA 1.800.360.7255 toll free (Only in US) 1.516.484.5400 phone 1.516.625.3610 fax Visit us on the Web at www.pall.com Pall Corporation has offices and plants throughout the world in locations including: Argentina, Australia, Austria, Belgium, Brazil, Canada, China, France, Germany, Hong Kong, India, Indonesia, Ireland, Italy, Japan, Korea, Malaysia, Mexico, the Netherlands, New Zealand, Norway, Poland, Puerto Rico, Russia, Singapore, South Africa, Spain, Sweden, Switzerland, Taiwan, Thailand, United Kingdom, United States, and Venezuela. Distributors are located in all major industrial areas of the world. © Copyright 2005, Pall Corporation. Pall, trademark registered in the USA. ABG-105-0305 , are trademarks of Pall Corporation. ® Indicates a Pall is a service mark of Pall Corporation.