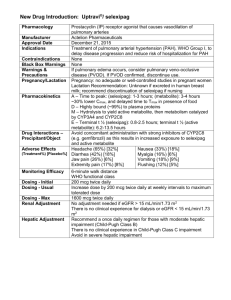
424 R&R and PHS-398 Specific Table Of Contents
advertisement
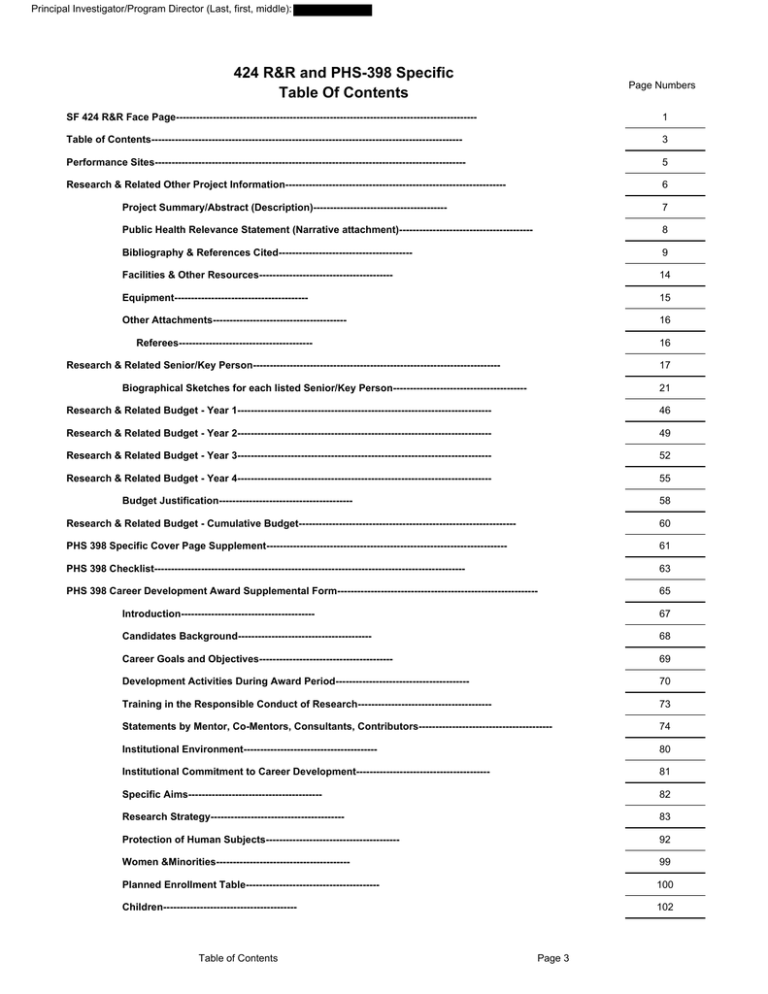
Principal Investigator/Program Director (Last, first, middle): 424 R&R and PHS-398 Specific Table Of Contents Page Numbers SF 424 R&R Face Page------------------------------------------------------------------------------------------ 1 Table of Contents--------------------------------------------------------------------------------------------- 3 Performance Sites--------------------------------------------------------------------------------------------- 5 Research & Related Other Project Information------------------------------------------------------------------ 6 Project Summary/Abstract (Description)---------------------------------------- 7 Public Health Relevance Statement (Narrative attachment)---------------------------------------- 8 Bibliography & References Cited---------------------------------------- 9 Facilities & Other Resources---------------------------------------- 14 Equipment---------------------------------------- 15 Other Attachments---------------------------------------- 16 Referees---------------------------------------- 16 Research & Related Senior/Key Person-------------------------------------------------------------------------- 17 Biographical Sketches for each listed Senior/Key Person---------------------------------------- 21 Research & Related Budget - Year 1---------------------------------------------------------------------------- 46 Research & Related Budget - Year 2---------------------------------------------------------------------------- 49 Research & Related Budget - Year 3---------------------------------------------------------------------------- 52 Research & Related Budget - Year 4---------------------------------------------------------------------------- 55 Budget Justification---------------------------------------- 58 Research & Related Budget - Cumulative Budget----------------------------------------------------------------- 60 PHS 398 Specific Cover Page Supplement------------------------------------------------------------------------ 61 PHS 398 Checklist--------------------------------------------------------------------------------------------- 63 PHS 398 Career Development Award Supplemental Form------------------------------------------------------------ 65 Introduction---------------------------------------- 67 Candidates Background---------------------------------------- 68 Career Goals and Objectives---------------------------------------- 69 Development Activities During Award Period---------------------------------------- 70 Training in the Responsible Conduct of Research---------------------------------------- 73 Statements by Mentor, Co-Mentors, Consultants, Contributors---------------------------------------- 74 Institutional Environment---------------------------------------- 80 Institutional Commitment to Career Development---------------------------------------- 81 Specific Aims---------------------------------------- 82 Research Strategy---------------------------------------- 83 Protection of Human Subjects---------------------------------------- 92 Women &Minorities---------------------------------------- 99 Planned Enrollment Table---------------------------------------- 100 Children---------------------------------------- 102 Table of Contents Page 3 Principal Investigator/Program Director (Last, first, middle): Resource Sharing Plan---------------------------------------- Table of Contents 103 Page 4 Principal Investigator/Program Director (Last, first, middle): Project Summary: Candidate: , MD, is an Assistant Professor in Pulmonary and Critical Care at the University of Texas Southwestern Medical Center in Dallas, TX. She seeks support for career development to emerge as an independent investigator in the area of pulmonary arterial hypertension. Environment: Mentorship will be provided by Dr. Milton Packer, an expert in cardiovascular clinical trials, with additional mentorship from Drs. Lewis Rubin, an expert in pulmonary hypertension, and Lance Terada, an expert on cell signaling. Research and Career Development: Dr. proposes two studies that will focus on the role for the serotonin transporter in pulmonary arterial hypertension (PAH). These two studies will test the hypothesis that the serotonin transporter is a key contributor to the development and persistence of elevated pulmonary pressures in patients with pulmonary arterial hypertension (PAH). The first study will be a randomized double-blind placebo controlled trial evaluating whether blockade of the serotonin transporter with fluoxetine, a selective serotonin reuptake inhibitor, will reduce pulmonary vascular resistance in patients with PAH. In her preliminary work involving open label use of fluoxetine, Dr. found that three months treatment led to improvement in cardiac index (p<0.05) and pulmonary vascular resistance (p=NS) compared with baseline. In the proposed clinical trial, 34 patients will be randomized to placebo or fluoxetine for six months. The primary endpoint will be change in pulmonary vascular resistance, and secondary endpoints will include other hemodynamic changes, exercise capacity, quality of life and safety. Dr. second study will evaluate whether use of stimulants and other medications that act as serotonin transporter substrates is associated with idiopathic PAH. This will be studied using a case-control design with 70 cases and 140 controls, and medication use will be determined by in-person survey. Dr. has previously shown that idiopathic PAH may be associated with use of methamphetamine, a stimulant with serotonin transporter substrate activity. Dr. proposes to supplement this practical training in clinical research with formal coursework in statistics, pharmacology and pulmonary vascular biology. This work addresses several National Heart Lung and Blood Institute strategic objectives, including (1) Improving the understanding of the molecular and physiological basis of health and disease, and (2) Improving the understanding of the clinical mechanisms of disease and thereby enabling better prevention, diagnosis, and treatment. Project Description Page 7 Principal Investigator/Program Director (Last, first, middle): Project narrative: This project aims to evaluate the role for the serotonin transporter in pulmonary arterial hypertension (PAH). Two studies are proposed: an evaluation of the hemodynamic effects of blocking the serotonin transporter with fluoxetine for six months, and a case-control study evaluating whether medications that increase serotonin transporter activity, such as methamphetamine, are associated with idiopathic PAH. These studies are relevant to public health because they may lead to novel therapies for idiopathic PAH, and because they may help us to better understand risk factors for this frequently fatal disease. Public Health Relevance Statement Page 8 Principal Investigator/Program Director (Last, first, middle): Facilities and Other Resources Laboratory and Animal: Not applicable. Clinical: Hospital, Catheterization Lab, Internal Medicine and Pulmonary Hypertension Clinics: St. Paul University Hospital is a 271 bed hospital located in Dallas County on the UT Southwestern Medical Center campus. This campus includes the medical school, research buildings, clinical buildings and two other hospitals (Parkland Memorial, Dallas’ County Hospital, and Zale Lipshy University Hospital). The cardiac catheterization laboratory is within St. Paul Hospital and includes three cardiac catheterization rooms. The Pulmonary Hypertension and the Internal Medicine Clinics are located within the POB building which is physically attached to the hospital. The Pulmonary Hypertension Clinic is supported by three nurses and two nurse practitioners, and patient care visits are staffed by four physicians and rotating pulmonary fellows. The pulmonary hypertension program provides care for more than 300 idiopathic and connective tissue disease PAH patients and more than 1000 pulmonary hypertension patients overall, making it one of the ten largest programs in the country in patient volume. More than 250 new patients seen yearly. Pulmonary hypertension research is conducted out of the same area but using a separate team of experienced research coordinators. This includes a pulmonary hypertension research manager / regulatory expert (Mary Ann Byerly), three full-time research coordinators and one research assistant. There are currently twelve active protocols including both industry and NIH sponsored studies, and several more that are under review by the IRB. The patient population combined with the experienced research personnel provides an ideal environment for clinical research. Clinical: Database: Screening for clinical trials is facilitated by an electronic database that contains diagnosis, catheterization and current medication information as well as other clinical characteristics. Clinical: Research sites: Patients enrolling in the clinical trial will be enrolled through the pulmonary hypertension clinic, described above. Catheterization procedures will take place in the adjacent hospital. For the case-control study, idiopathic PAH and other PH patients will be recruited through clinic, while normal controls will be recruited from the Internal Medicine clinic, also located in the POB building. This location facilitates access for the researchers and coordinators. Offices and computers: Physician and research staff offices are located within the St. Paul POB II building, floor one, pulmonary division offices. This includes three 150 sq. foot offices (PI office and two research offices), and a research cubicle area for an administrative assistant, a research assistant and one coordinator. Offices and cubicles all include computers purchased in the last three years, internet access, and printer and phone access. Other resources: As noted in the budget section and in the support letters, several additional sources of support will be available during the period of the award. First, additional research coordinator support, in excess of that covered by the award, will be provided by the Pulmonary Division. Second, the Department of Clinical Sciences (through CTSA mechanisms) will provide matching funds for the annual $30,000 allocation from the K23 to support the conduct of Dr. research. For other details on resources, see also the description of the institutional environment and the institutional commitment to the candidate’s career development sections. Facilities Page 14 Principal Investigator/Program Director (Last, first, middle): Equipment: Not Applicable Equipment Page 15 Principal Investigator/Program Director (Last, first, middle): This application will have letters from the following Referees: 1. Richard Channick, M.D. Director, Pulmonary Hypertension Program Associate Professor of Medicine Massachusetts General Hospital Boston, MA 2. David Badesch, M.D. Clinical Director, Pulmonary Hypertension Center Professor of Medicine University of Colorado Denver, CO 3. Clyde W. Yancy, M.D. Chief, Division of Cardiology Professor of Internal Medicine Northwestern University Feinberg School of Medicine Chicago, Il Referees Page 16 Principal Investigator/Program Director (Last, first, middle): BIOGRAPHICAL SKETCH Provide the following information for the Senior/key personnel and other significant contributors. Follow this format for each person. DO NOT EXCEED FOUR PAGES. NAME POSITION TITLE Assistant Professor of Medicine eRA COMMONS USER NAME (credential, e.g., agency login) EDUCATION/TRAINING (Begin with baccalaureate or other initial professional education, such as nursing, include postdoctoral training and residency training if applicable.) DEGREE INSTITUTION AND LOCATION MM/YY FIELD OF STUDY (if applicable) The University of Texas, Austin UT Southwestern Medical School, Dallas UT Southwestern Medical Center, Dallas BA MD Residency 05/92 06/96 06/00 University of California, San Diego Fellowship 06/05 MSc 07/11 UT Southwestern Graduate School, Dallas Biology Medicine Internal Medicine Pulmonary and Critical Care Clinical Sciences A. Personal Statement The goal of the proposed research is to investigate the role for the serotonin (5HT) transporter in pulmonary arterial hypertension (PAH). Two main studies will be completed: an evaluation of the hemodynamic changes in PAH with 24-weeks treatment with fluoxetine, and a case-control study evaluating whether use of medications that increase serotonin transporter activity are associated with idiopathic PAH. I have already completed a pilot study that evaluated two serotonin pathway medications in PAH (fluoxetine and reserpine; see preliminary data); based on these results my current plan is to move forward with a randomized, double-blind placebo controlled clinical trial that will evaluate efficacy of fluoxetine compared with placebo. Separately, I will also perform a case-control study evaluating whether there is an association between medications that increase serotonin signaling and idiopathic PAH. This project expands on an earlier case-control study that I completed at UCSD, where I found an association between stimulants such as methamphetamine and idiopathic PAH. I am well-qualified to complete these studies, based on my background in pulmonary hypertension and my relationship with an exceptional team of mentors. My experience in multi-center clinical trials in particular has prepared me to carry out clinical studies, but I now need additional training in order to design and conduct studies of my own. I have am working with an outstanding team of mentors (Drs. Packer, Rubin and Terada), and I also have three advisors who will help with specific aspects of the project, including experts on survey development (Ira Bernstein, PhD), epidemiology (Robert Haley, MD) and statistics (Joan Reisch, PhD). Throughout this training period, I will also complete coursework in pulmonary vascular biology, statistics and epidemiology. In summary, I am optimally situated to complete the proposed research, based on my extensive background in pulmonary hypertension combined with an exceptional team of highly skilled mentors and advisors. B. Positions and Honors. Positions and Employment 1997-2000 Internal Medicine Residency. UT Southwestern, Dallas, TX. 2000-2002 Internal Medicine Practice. Baylor Hospital, Irving, Texas. 2002-2005 Pulmonary Fellowship. University of California. San Diego, CA. 2005-2006 Southwest Pulmonary Associates, St. Paul University Hospital, Dallas, TX. 2007Assistant Professor of Medicine. UT Southwestern Medical School, Dallas, Tx. 2008-2010 Associate Director, UT Southwestern Pulmonary Hypertension Program, Dallas, Tx. 2010Director, UT Southwestern Pulmonary Hypertension Program, Dallas, Tx Other Experience and Professional Membership American Medical Association American Thoracic Society American College of Chest Physicians Biosketches Page 21 Principal Investigator/Program Director (Last, first, middle): Pulmonary Hypertension Association Honors, Awards and Appointments University of Texas Academic Scholarship (1989-1992) College of Natural Science Dean’s Honor List, UT Austin (1989-1992) Southwestern Medical Foundation Academic Scholarship (1992-1996) Finalist, Alfred Soffer Research Award (ACCP Original Research Award, 2007) KL2 Clinical Scholar (2008-2011) Editorial Board, Advances in Pulmonary Hypertension (2010-current) Guest Editor, Fall 2011: Women's Issues in Pulmonary Hypertension Steering Committee, Griphon (Phase III Trial; Actelion, 2010-current) Steering Committee, Epitome (Phase IV Trial; Actelion 2010-Current Publication Committee, Thelin (Phase III Trial; Pfizer, 2010-current) Board Certification 1. Critical Care Medicine 2. Pulmonary Medicine 3. Internal Medicine 2006-2016 2005-2015 2001-2011 C. Peer-reviewed publications (published and submitted in chronological order). Peer Reviewed Research Manuscripts 1. Chin KM, Channick RN, Rubin LJ. Is Methamphetamine Use Associated with Pulmonary Arterial Hypertension? Chest 2006;130(6):1657-1663. 2. Chin KM, Channick RN, Kim NH, Rubin LJ. Central Venous Blood Oxygen Saturation Monitoring in Patients With Chronic Pulmonary Arterial Hypertension Treated With Continuous IV Epoprostenol: Correlation With Measurements of Hemodynamics and Plasma Brain Natriuretic Peptide Levels. Chest 2007;132:786-92. 3. Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere Shortening in Familial and Sporadic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2008; 178: 729-737. PMC2556455 4. Chin KM, Kingman M, de Lemos JA, Warner JJ, Reimold S, Peshock R, Torres F. Changes in right ventricular structure and function assessed by cardiac magnetic resonance imaging in bosentan treated patients with pulmonary arterial hypertension. Am J Cardiology. 2008;101:1669-1672. 5. Chin KM, Channick RN, de Lemos JA, Kim NH, Torres F, Rubin LJ. Hemodynamics and epoprostenol use are associated with thrombocytopenia in pulmonary arterial hypertension. Chest, 2009;135:130-136. 6. Blalock SE, Matulevicius S, Mitchell LC, Reimold S, Warner J, Peshock R, Torres F, Chin KM. Long-term Outcomes with Ambrisentan Monotherapy in Pulmonary Arterial Hypertension. J Card Fail. 2010 Feb;16(2):121-7 7. Kingman MS, Tankersley MA, Lombardi S, Spence S, Torres F, Chin KM. Prostacyclin administration errors in pulmonary arterial hypertension patients admitted to hospitals in the United States: a national survey. J Heart Lung Transplant. 2010;29:841-6. 8. Link JJ, Glazer C, Torres FT, Chin KM. ICD Coding Changes Lead to Profound Declines in Reported Idiopathic Pulmonary Arterial Hypertension Mortality and Hospitalizations: Implications for Database Studies. Chest. 2011; 139:497-504. Book Chapters and Review Articles (selected from a total of 8) 1. Chin KM, Channick RN, Rubin LJ. Pulmonary Hypertension: Diagnosis and Treatment. In Manual of Clinical Problems in Pulmonary Medicine, RA Bordow, AL Ries, TA Morris, eds., Lippincott Williams & Wilkins, 2004. 2. Chin K, Channick R. Bosentan. Expert Rev Cardiovascular Ther. 2004;2:175-182. 3. Chin KM, Kim HS, Rubin LJ. The Right Ventricle in Pulmonary Hypertension. Coron Artery Dis. 2005;16:13-18. 4. Chin K, Fedullo P. Chronic Thromboembolic Pulmonary Hypertension. In Pulmonary Vascular Disease. Mandel J, Taichman D, eds, 2006. 5. Chin KM, Rubin LJ. State of the Art: Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2008;51:15271538. Biosketches Page 22 Principal Investigator/Program Director (Last, first, middle): 6. Chin KM, Torres F, Rubin LJ. Idiopathic and Heritable Pulmonary Hypertension: Introduction to Pathophysiology and Clinical Aspects. In Pulmonary Circulation. LJ Rubin, AJ Peacock, eds., 2011. 7. Chin KM, Torres F. Surgical Treatments. In Pulmonary Hypertension. A Patient’s Survival Guide. RJ Oudiz, MD, ed. 2011. D. Research Support Ongoing Research Support Gilead: Ambrisentan in CHF Torres (PI) 2008-current Single center randomized controlled clinical trial studying the effects of ambrisentan vs. placebo in patients with heart failure with preserved ejection fraction plus out of proportion pulmonary hypertension. Role: Co-PI United Therapeutics: Freedom (3 studies) Torres (PI) 2008-current Multicenter phase III study of oral treprostinil in pulmonary arterial hypertension. Role: Sub-I Cicletenine Torres (PI) Multicenter phase III study of cicletenine in pulmonary arterial hypertension. Role: Sub-I 2009-current Lung Rx: Beraprost phase II Torres (PI) Multicenter phase II study of beraprost in pulmonary arterial hypertension. Role: Sub-I 2009-current Bayer: Chest Torres (PI) 2009-current Multicenter study of oral BAY-635 in chronic thromboembolic pulmonary hypertension Role: Sub-I Actelion: Seraphin (PI) Randomized double-blind placebo controlled trial of ACT-064992 in PAH Role: PI 2009-current Bayer: Patent Torres (PI) Multicenter study of oral BAY-635 in pulmonary arterial hypertension Role: Sub-I 2009-current Actelion: Prospect (PI) 2010-current Open label study of long-term outcomes with actelion epoprostenol in pulmonary arterial hypertension Role: PI Actelion: Griphon (PI) Randomized study of ACT-293987, a novel prostacyclin receptor agonist Role: PI Select Completed Research Support United Therapeutics: Treinh Torres (PI) Inhaled treprostinil for pulmonary hypertension with ILD Role: Sub-I 2010-current 2008-2009 Actelion: Compass 3 Torres (PI) 2006-2010 Bosentan +/- sildenafil in the treatment of PAH; effects on cardiac MRI and hemodynamics Role: Sub-I Actelion: Inhale 15 (PI) 2008-2011 Multicenter randomized controlled clinical trial studying the effects of changing to the power 15 disc among patients receiving inhaled iloprost for PAH. Biosketches Page 23 Principal Investigator/Program Director (Last, first, middle): Role: PI Pfizer: Sitaxsentan (PI) 2009-2011 Randomized double-blind placebo controlled study of a novel PDE-5 inhibitor on hemodynamics in IPAH Role: PI Pfizer: Sitaxsentan +/- sildenafil (PI) 2009-2011 Randomized controlled study to follow the earlier sitaxsentan study; in this follow-up all patients received sitaxsentan and are randomized to sildenafil vs. placebo. Role: PI Actelion: Epitome (PI) Randomized open-label study of Flolan vs. Actelion Epoprostenol Role: PI 2009-2011 Gilead: Athena Torres (PI) 2009-2011 Open label study investigating the addition of ambrisentan in patients with PAH receiving a PDE-5 inhibitor Role: Sub-I Gilead: Artemis PH Torres (PI) 2009-2011 Multicenter study of ambrisentan in pulmonary hypertension associated with IPF Role: Sub-I *PI reflects center PI for industry studies; all are multi-center except the Ambrisentan in CHF study which is single center and investigator initiated NIH Research Support - Completed 1T32HL076126-01 Wagner (PI) NIH National research Service Award at UCSD (T32) Role: Post-doc 7/1/04-6/30/05 1F32HL082216-01 (PI) 12/2/05: terminated Individual Ruth L. Kirschstein National Research Service Award. Project Title: Are Stimulants Risk Factors for Pulmonary Arterial Hypertension? University of California, San Diego. Grant approved; not awarded due to relocation to Dallas. Role: PI 5 UL1 RR024982-03 Packer (PI) 08/01/09-07/31/10 The goals of this pilot award obtained through the North and Central Texas Clinical and Translational Science Initiative are to evaluate the safety and efficacy of serotonin transporter blockade with fluoxetine and serotonin depletion with reserpine in pulmonary arterial hypertension. Role: Co-Investigator (Recipient of Pilot Award) 1 R01 HL078946-04 Benza (PI) 9/1/05-7/31/10 Pharmacogenomics in Pulmonary Arterial Hypertension The goals of this study are to determine whether disease specific polymorphisms (serotonin transporter, PAI BMPR2, SMAD4, among others) are associated with response to endothelin receptor blocker therapy. Role: Co-investigator (Center PI) 5 KL2 RR024983-04 Packer (PI) North and Central Texas Clinical and Translational Science Initiative (KL2). Role: Clinical Scholar Biosketches 7/1/08-6/30/11 Page 24 Principal Investigator/Program Director (Last, first, middle): Introduction to the revised application: This is a revised application for the K23 proposal, Serotonin Signaling in PAH, that was originally submitted 2/12/2010. I would like to thank the reviewers for their suggestions and critiques. Because of major changes that have been undertaken, the majority of text in the application is either new or revised, and thus changes are not marked within the application. The original proposal received a priority score of 40. Individual scores ranged from 1-3 for the candidate, 1-2 for both the career development plan and mentors, 2 for the environment and 2 to 5 for the research plan. The biggest concern, cited by both reviewers 1 and 2, related to feasibility, particularly for the clinical trial. This included both the limited amount of preliminary data and the dependence of aims 2 and 3 on aim 1. To address these concerns, I have now completed enrollment in an open label clinical pilot study (N=9, essentially the prior aim 1), demonstrating my ability to carry out the proposed study. Importantly, the pilot clinical trial also demonstrated hemodynamic improvement with fluoxetine alone. Therefore, although our original hypothesis was that we would need to block both the serotonin transporter and reduce serotonin receptor signaling in order to improve hemodynamics, we now propose that blocking the serotonin transporter for 6 months therapy with fluoxetine alone, added to stable background therapy, will lead to improvement in pulmonary vascular resistance (PVR) vs. placebo. Reviewer 3 also provided several additional suggestions, including assessment of the durability of any changes in PVR in the clinical trial, consideration of studies of the platelet-serotonin axis, and consideration of the use of pulmonary hypertension (PH) controls, in addition to normal controls in the case-control study. We agree. The long-term durability of the response is a particularly important question, and in fact we have reported this type of data for participants at our center who completed clinical trials75-76; we will ensure that similar follow-up data is collected in this study as well. For the platelet-serotonin axis question, we have added a sub-study to the clinical trial which will involve ELISA tests to measure platelet and endothelial activation (table 5 in the research plan). We do not plan to perform in vitro platelet aggregation tests or studies of circulating serotonin levels because both of these types of study have been inconsistent in PH and may not be informative. Finally, for the casecontrol study, we have clarified inclusion-exclusion criteria for both cases and controls and we have added a PH control group to the already planned normal control group. The number of aims is also reduced to two. The former Aim 1 has been completed (pilot study), and the former Aim 3 (5-HIAA measures) is now listed as a sub-study in table 4 in the research plan. Another change to the proposal is a revised career development plan. The current proposal includes more training in pulmonary vascular biology as well as a basic science pharmacology course, as suggested by several reviewers. A clarification about what will be gained by the proposed coursework vs. the completed UTSW Clinical Scholars curriculum is also provided. In particular, this includes details about the advanced statistics and epidemiology courses offered in the School of Public Health at the University of North Texas. Finally, a clearer description of how my time will be protected is provided, as detailed in a letters from Dr. Fernando Torres, now serving as Head, Lung Transplantation and Pulmonary Hypertension Programs (letter specifically requested by reviewers) and in the mentor letter from my Division Chief, Dr. Lance Terada. I appreciate the opportunity to resubmit this application, and look forward to your reviews. Introduction Page 67 Principal Investigator/Program Director (Last, first, middle): Candidates’s Background: I developed an interest in pulmonary arterial hypertension (PAH) during my Internal Medicine Residency at the UT Southwestern Medical Center (UTSW). During that time, I cared for a young patient who did very poorly despite our attempts at treatment. The need for better treatment options combined with my interest in cardiopulmonary physiology led me to pursue PAH as a specialty. I completed my pulmonary and critical care fellowship at UC San Diego (UCSD), chosen for its extensive pulmonary vascular disease program and its status as one of the top overall pulmonary programs in the country. My most important accomplishments during this time were under the direction of my primary mentor, Dr. Lewis Rubin, an internationally recognized expert in PAH. With his guidance, I completed three clinical research projects and I participated as a sub-investigator on several clinical trials. I was also nominated for and funded for one year by UCSD’s T32 training grant, selected by my program director based on my accomplishments and potential as a clinical investigator. I also submitted an F32 NRSA fellowship application that ultimately received favorable scores, but while waiting on the somewhat delayed funding decision, I applied for and accepted a position in PAH in Dallas. Although not initially an academic position, the hospital and PAH clinic were owned by my medical school and residency alma mater, UTSW, and as I anticipated, I was asked to join UTSW as an Assistant Professor two years later, in 2007. Transition to a Clinical Research Track: Wanting to expand my involvement in research, in 2008 I applied for and was accepted into UTSW’s highly competitive Clinical Scholars Program, through which I recently completed a master’s degree. The Scholars program provided me with one year of salary support (through a KL2 mechanism) and three years of other support including coursework and training in clinical research. It has also helped me to develop relationships with collaborators and mentors with whom I continue to work. Since joining UTSW, I have also published seven manuscripts and two book chapters on pulmonary hypertension, I have served as a site principal investigator on seven multi-center clinical trials, and I received a CTSA pilot award that allowed me to design and carry out a single center pilot study (open label) in PAH. These experiences have provided me with a better understanding of the mechanics of clinical trials, but I now want to design and lead clinical trials of my own. Candidates Background Page 68 Principal Investigator/Program Director (Last, first, middle): Career goals and objectives: My long-term goal is to develop an independent career in pulmonary hypertension clinical research. Specifically, I am interested in developing novel therapies for PAH, in developing better markers of prognosis in PAH and in using clinical research to better understand the underlying disease process. To accomplish these goals, I will need to be able to design, conduct and participate in the analysis of robust clinical studies of my own design. Toward this end, my training plan has been designed to strengthen specific areas of relative weakness in my training to date. These areas of focus will provide me with: 1) deeper training in advanced statistics and epidemiology (objective 1); 2) improved ability to anchor clinical studies to basic scientific advancements (objective 2); 3) enhanced writing and speaking skills (objective 3); and 4) greater hands on experience in designing and conducting clinical studies (objective 4). Therefore, the Mentored Clinical Scientist Development Award will serve as an effective training vehicle to complement rather than replicate my existing training. With respect to coursework, I will draw heavily from the University of North Texas Health Science Center School of Public Health. This program is one of the partner institutions in our CTSA, chosen because of its emphasis on epidemiology and population statistics. These MPH level courses will balance the more clinically focused UTSW Master’s curriculum that I completed which covered only introductory level statistics and epidemiology. Additionally, I will also take advantage of the expertise available in UT Southwestern’s Pulmonary Vascular Biology Division and School of Biomedical Sciences to expand my skillset in pulmonary vascular biology and advanced pharmacology. This combination of skills is important to my future work in clinical trials in PAH. Career Goals and Objectives Page 69 Principal Investigator/Program Director (Last, first, middle): Career Development: In this grant application, I have proposed a research plan that will help me to acquire the knowledge and skills to conduct a wide range of clinical studies in PAH. Specific objectives are shown table 2 and include a particular focus on training in statistics, epidemiology, pharmacology and pulmonary vascular biology. Nine classes (25 credits) will be taken; this allows for one course to be taken most semesters (fall / spring / summer) while reserving most of my time to be spent on clinical research work. Table 1 Fluoxetine Trial Case-Control Study Course-work Grant Applications Year 1 Enroll 10 Enroll 100 4 classes (8 hrs) Year 2 Enroll 11 Enroll 100 3 classes (8 hrs) Year 3 Enroll 11 Enroll 10; analysis 2 classes (6 hrs) Case-Control Study Year 4 Enroll 2; analysis 1 class (3 hrs) Clinical Trial All courses are semester-long except for the year long pulmonary vascular biology course, and all courses are Master’s level or higher. Courses to be completed at UNT (“NT” below) include Nonparametric Statistics, Clinical Trial and Survival Analysis, and a Statistics Lab which will help me complete my analyses for the randomized controlled clinical trial of fluoxetine (Aim 1 of the research plan), plus a Regression Analysis and Applied Data Analysis in Epidemiology which will help me conduct and analyze the case-control study of medication use in PAH (Aim 2 of the research plan). Courses to be taken through the Clinical Sciences Graduate School at UTSW (“SW” below) include Pharmacology, to cover pharmacokinetics, distribution, metabolism/elimination and drug-drug interactions, Developing and Validating Measures, to cover the design and use of surveys and scales such as those used in both the clinical trial and the case-control study, and Grant Writing, to help me polish my writing skills. Finally, courses taken through UTSW’s School of Biomedical Sciences include Pulmonary Vascular Biology, focusing on current and classic papers related to vascular cell signaling, molecular physiology of the endothelium and vascular pathobiology, and Mechanisms of Drug Action covering the molecular basis of pharmacological selectivity, rational drug design, peptides, proteins and RNA as drugs and gene therapy. In addition to helping me to carry out and analyze the studies in the current application, these courses will also be invaluable to my work on multi-center clinical trials, particularly as I become involved in the design and analysis of these studies. The five offsite courses at UNT are in Fort Worth; importantly, these courses are offered as a 2-3 hour block one day per week, minimizing interruptions to my research. Additionally, because I live on the west side of Dallas, the commute will be less than 20 minutes. Table 1: Specific objectives Year Objective 1: Increase my understanding of clinical research, statistics and epidemiology o 1 2 3 Complete coursework in: o Year 1: Statistics Lab (NT5314), Regression Analysis (NT5312), Pharmacology (SW5203) o Year 2: Nonparametric Statistics (NT316), Developing and Validating Measures (SW5201) o Year 3: Applied Data Analysis in Epidemiology (NT5314), Survival Analysis (NT6318) Attend a weekly clinical research work in progress seminar and a monthly career development seminar sponsored by the K30 clinical scholars program Objective 2: Enhance my knowledge of basic science and pulmonary vascular biology o Complete UTSW’s Pulm. Vascular Biology (PVB) and Mechanisms of Drug Action classes Meet bimonthly with Dr. Lance Terada, basic science mentor, to place clinical hypotheses and data in the context of current molecular science; attend weekly PVB Work in Progress meeting Objective 3: Enhance my ability to eloquently present my research data o o Complete course on Grant Writing & Funding Strategies (SW5106) o Present research locally on an annual basis at the Clinical Science Dept’s Clinical Research Forum and the PVB Work in Progress Conferences; submit abstracts annually for presentation at national meetings such as the American Thoracic Society and American Heart Association o Complete grant applications, anticipated to consist of (1) A multi-center case-control study investigating stimulants and other medications in PAH, and (2) A multi-center randomized controlled trial evaluating fluoxetine in PAH, either as R01 or potentially R21 for the trial o Submit 1-2 manuscripts per year, based on the projects included in this application Objective 4: Increase my hands-on experience in designing and conducting clinical research o Complete the randomized placebo controlled clinical trial described in Aim 1 o Complete the case-control study of medication use in PAH described in Aim 2 Meet weekly with Dr. Packer to discuss clinical research progress and meet regularly with Drs. Development Activities During Award Period Page 70 o o 4 Principal Investigator/Program Director (Last, first, middle): Rubin, Haley (epidemiology), Bernstein (survey development) and Reisch (statistics) Abbreviations: NT: University of North Texas classes. SW: UT Southwestern classes Mentors Dr. Milton Packer will serve as my primary mentor for the duration of the award. Dr. Packer is currently Professor and Chair for the Department of Clinical Sciences at UT Southwestern Medical Center, the director of the UTSW KL2 Clinical Scholars Program, and the Director of UTSW’s Center for Heart Failure Research. Dr. Packer has extensive experience in cardiology clinical trials, and an accomplished mentoring career. The majority of his former mentees have gone on to have successful academic careers. Dr Packer agreed to serve as my mentor in 2009, and we have subsequently worked together on multiple projects including an intramural grant application that was funded, a clinical trial in pulmonary hypertension, and two papers that were published in 2010-2011. Dr Packer and I will meet weekly or more often, as needed, ensuring my successful transition to an independent investigator. Under his mentorship, I will identify, based on the results of this project, promising directions for future research including future R01 grants. Dr. Lewis Rubin will serve as a secondary mentor providing advice particularly with regards to conducting clinical trials in pulmonary hypertension. Dr. Rubin is an Emeritus Professor of Medicine at UCSD and is the Emeritus Director of the Pulmonary Hypertension Program at UCSD. He has prior NIH and foundation grant funding, and has numerous prior mentees who went on to have successful academic careers. Dr. Rubin served as my primary mentor during my fellowship at UCSD, and he has continued to provide me with valuable advice and direction during the five years since I left UCSD. We meet in person at least three times per year, usually in conjunction with a national meeting or at an investigators meeting for a study, and this will continue throughout the award. In addition, throughout the period of the award, Dr. Rubin and I will speak monthly by phone and will continue to correspond regularly by email. Dr. Lance Terada will serve as a secondary mentor, focusing on basic science. Dr. Terada is a Professor of Internal Medicine, Chief of the Division of Pulmonary and Critical Care at UTSW, and is an experienced basic scientist working in pulmonary vascular biology. His research interests are in the area of endothelial cell signal transduction, with a focus on the role for reactive oxygen species and other factors in mediating cell proliferation and signals of cell death. Dr. Terada and I will meet every 2 weeks, with a focus on pulmonary vascular biology, the basic science implications of my research, and opportunities for collaboration. Advisors: I also have three outstanding advisors who will assist me with more focused aspects of these studies. Robert Haley, MD will serve as an advisor and epidemiology consultant, providing frequent feedback on the case-control study. Dr. Haley is a Professor of Internal Medicine and Director of the Division of Epidemiology in the Internal Medicine Department at UTSW. Joan Reisch, Ph.D will provide statistical advice and support. Dr. Reisch has a Ph.D. in statistics, and is a Professor of Clinical Sciences in the UTSW Department of Clinical Sciences. Dr. Ira Bernstein, Ph.D will provide advice on survey development for the case-control study. Dr. Bernstein is a Professor of Clinical Sciences at UTSW and his research involves the development, analysis, validation, and application of scales (psychometric theory). Advisory committee: In addition to regular, individual meetings with my mentors and advisors, Dr. Packer will also chair a career advisory committee that includes Dr. Terada and Dr. Rubin; Dr. Rubin will join via teleconference. This committee will meet every 4 months. Specific activities during committee meetings will include, but are not limited to: 1) review of research activity progress, 2) review of career training progress and activity, 3) review of timeliness and quality of scholarly production, and 4) suggestions for improvement of research plans and analyses. Specific targets that should be met include enrollment of 12 patients per year in the clinical trial (complete by early year 4), enrollment of 105 patients per year in the case-control study (complete by end of year 2), publish 1-2 research papers per year, and by year 3 begin grant applications Clinical responsibilities: During the period of the award, my clinical responsibilities will encompass 20% of my time and administrative responsibilities 5%. This will be feasible because we currently have four pulmonary hypertension physicians: myself, Dr. Fernando Torres (see letter), Dr. Sonja Bartolome, Associate Director of the PAH program, and Dr. Rosechelle Ruggiero, all three of whom are able to shift to a higher percentage effort in pulmonary hypertension (by reducing general pulmonary obligations). Additionally, Dr. Torres, Head of the Combined Pulmonary Hypertension and Lung Transplant Center, will continue to oversee much of the administrative work for the pulmonary hypertension program so as to minimize my administrative duties. Participating in these clinical responsibilities is important to keep my clinical skills up to date and to my future career as a clinician investigator. Development Activities During Award Period Page 71 Principal Investigator/Program Director (Last, first, middle): Summary: In summary, with this training and experience, I will develop the skills required to achieve my career goals. This includes an improved understanding of statistics, epidemiology and pulmonary vascular biology through both coursework and clinical research projects. The sophistication of my research projects and my level of independence continues to progress, and my track record suggests that with additional training, I will obtain the skills needed to become an independent investigator. Development Activities During Award Period Page 72 Principal Investigator/Program Director (Last, first, middle): Training in the responsible conduct of research: Because patient safety and confidentiality are an integral part of clinical research, I have completed training in Good Clinical Practices, and I have completed a semester long course in Public Policy Considerations in Research, which covers regulatory requirements in clinical research and discussed (1) The Belmont Report; (2) Title 45 Code of the Federal Regulations Part 46; (3) Responsibilities of Investigators in Human Research. I will also complete during the first year of the award a year-long, twice monthly Ethics in Clinical Research course that will cover informed consent, privacy and confidentiality, clinical equipoise and other ethics topics through bimonthly small group discussions. Continued training during the period of the award will include periodic completion of computer based certifications on Patients’ Rights and HIPAA compliance that are required by UT Southwestern researchers, and participation in Ethics grand Rounds (1 hr monthly) at UT Southwestern. Finally, Dr. Packer, my primary mentor, will oversee this training and will also incorporate ethics training into our regular weekly meetings. Training in the Responsible Conduct of Research Page 73 Principal Investigator/Program Director (Last, first, middle): A. Statement by Sponsor, Milton Packer, MD: I am pleased to provide my strongest support for for her application for a K23 Mentored Patient Oriented Research Career Development Award. As I will describe more fully below, has both the scientific skills and personal attributes that will, without a doubt, allow her to excel and develop into an independent investigator. I currently serve as the Chair of the Department of Clinical Sciences at the University of Texas Southwestern (UTSW) Medical School. This department is the academic home for our NIH Clinical and Translational Science Award, and I was formerly the overall Principal Investigator for this award. The primary mission of this department is to support the career development of clinical investigators. Over the past twenty-two years I have served as a mentor to more than 20 fellows and junior faculty, nearly all of whom remain in academic medicine and have emerged as independent clinical investigators. I have also served on NIH and Howard Hughes Medical Institute study sections for career development awards. My own research career is focused on understanding the mechanisms and evaluating new treatments for cardiovascular disease, particularly heart failure. In that capacity, I have extensive personal experience in clinical research; have published extensively in my area of focus; have been the overall Principal Investigator for numerous large-scale clinical trials, and have an appointment at FDA as one of their primary consultants in clinical trial design and analysis. I currently teach the principles of clinical research design and analysis in several courses at the UTSW Graduate School of Biomedical Sciences, for which I serve as the course director. I am also the lead investigator for our NIHfunded KL2 Clinical Scholars Program. Although I interact closely with all of the Scholars that are currently in our KL2 program, is the only trainee for whom I am currently acting as the primary mentor. I have known since she began her clinical sciences training through my role as the Director of the KL2 Program, through the year-long series of clinical research courses that I teach, and over the last 2 years, as her primary mentor. I therefore believe that I am well qualified to speak to her career and research potential. There is little doubt that - based on her knowledge, skill set and overall determination is the most qualified K23 applicant that I have worked with during the past decade. She is perfectly poised to become a future leader in pulmonary vascular medicine: she has the personal traits, skills and training to move this field forward and is working in an environment that will be highly supportive and conducive to her growth. B. Applicant’s Previous Accomplishments and Investigative Potential: came to UTSW with an outstanding academic record. She received competitive academic scholarships for both her undergraduate work at the University of Texas at Austin and for her medical school training at UTSW in Dallas. She stayed at UTSW for her Internal Medicine training, followed by the completion of a Pulmonary and Critical Care fellowship at the University of California, San Diego (UCSD), both very competitive programs. During her fellowship training, she showed a strong potential for a successful academic career. She was chosen to receive funding for one year of her fellowship through an NIH T32 sponsored training grant, and she successfully completed three clinical research projects, including the case-control study that supplies some of the background information for this proposal. During her final fellowship year, she also successfully competed for a Ruth L Kirchstein National Research Service Award (NIH F32); she ultimately chose not to accept this award, as an opportunity to pursue a pulmonary hypertension position affiliated with UTSW became available. Dr. began her first full-time faculty position at UTSW in 2007, serving initially as a full-time clinician in pulmonary hypertension. Based on her potential and her desire to pursue a research career, she transitioned to the clinical research track in 2008. She was given full departmental support for this change, and at the same time she was also accepted into our NIH KL2 sponsored Clinical Scholar Program. This competitive program, for which I serve as director, provides intensive, individualized training in clinical research and career development, and culminates in a Masters of Science in Clinical Sciences degree through the UTSW Graduate School of Biomedical Sciences. The intent and design of the KL2 program is to provide initial training and partial support for up to 3 years for extremely promising, early career clinical investigators. According to the plan submitted and approved by the NIH, our KL2 program is not intended to provide training and funding for an individual’s entire transition period to becoming an independent clinical investigator. We envision that it should take 6 years for an early career clinical investigator to become independently funded, and thus, our three-year KL2 program is intended to provide only early support for this period. Accordingly, our KL2 program requires all KL2 Clinical Research Scholars to submit and compete successfully for a K award (K08 or K23). Importantly, whereas the KL2 is an institutional award, the K23 represents an award in the investigator’s own right, which facilitates their transition to an RO1 or similar award far more effectively than a KL2 award alone. Statements by Mentor, Co-Mentors, Consultants, Contributors Page 74 Principal Investigator/Program Director (Last, first, middle): However, application for a K23 award is not a programmatic formality. We believe that this additional support comes at a critical time, providing the opportunity to acquire important new skills — particularly in the areas of advanced statistical methods, epidemiology and pulmonary vascular biology. These are essential to her successful development as an independent clinical investigator. publication record includes eight peer-reviewed studies, five book chapters and three review articles, and she is the first or senior author on all but one of these sixteen manuscripts. She has been recognized for her research, winning an ACCP Alfred Soffer Research Finalist Award for the abstract and oral presentation of one of her studies, and she has also presented the results of her research at several national meetings. Additionally, she has also been recognized for her expertise within her own subspecialty, with invitations to speak at the national meeting of the Pulmonary Hypertension Resource Network, and the American College of Chest Physicians. One of the reviewers of an earlier K23 application wondered why and I had not been co-authors on any scientific papers. The reviewer imagined that the lack of such publications might indicate a lack of meaningful mentoring or interaction. Nothing could be farther from the truth. The absence of my name on papers is intentional and has occurred upon my specific request. Since moving to Dallas seven years ago and being a leader in the career development of many young faculty, it has been apparent that co-authorship often unfairly dilutes the achievements of a rising star. It has therefore been my personal philosophy when mentoring young investigators to avoid co-authorship and the subsequent dilution of a young investigator’s track record of scholarly achievement. now seeks to address unanswered questions about the role for serotonin in pulmonary hypertension, using a case-control study and a clinical trial utilizing a novel strategy of serotonin transporter blockade. This latter study will allow her to both evaluate the hemodynamic and clinical effects of reduced serotonin transporter uptake in pulmonary hypertension. A proposal based on this idea was very well-received by the intramural grant review committee which reviews application for our NIH / NCRR sponsored Clinical and Translational Science Pilot Award; her open label pilot study was one of only three submissions funded at the higher $50,000 award level. has shown creativity in the project’s design, and has demonstrated the attention to detail required to keep this project on track. Specifically, she obtained an IND exemption, UTSW IRB approval, St. Paul University Hospital approval and then achieved full enrollment in the study all within the targeted time-frame. More importantly, having learned that her initial K23 application was well-received but not funded, did not become discouraged; instead, she redoubled her efforts. Specifically, using limited funds, she proceeded to carry out the early components of her originally proposed K23 research plan. In doing so, she developed a body of preliminary results which has allowed her to revise and refocus her research plan; these improvements are fully incorporated in the current K23 application. I should state emphatically that her undiminished determination to pursue her original research proposal speaks volumes, not only about her personal character and qualities, but also about the likelihood of her intermediate- and long-term success, if her revised K23 application is funded. C. Plan for the Candidate’s Training and Research Career Development: has had prior training in clinical research design and analysis through her coursework in the KL2 Clinical Scholars Program and through her fellowship training at UCSD. Additional training, mentorship and practical experience are now essential in order for her to transition from a promising junior faculty member into a fully independent investigator. Her career development plan includes leveraging exposure to the expertise and support of a world-class multidisciplinary group of mentors. Specifically, this will include mentoring with Dr. Lewis Rubin, Emeritus Director of the Pulmonary Hypertension Program, UCSD and Dr. Lance Terada, Director of the Pulmonary and Critical Care Division, UTSW, in addition to my own role as her sponsor and primary mentor. She is also working with an exceptional team of advisors who will provide focused advice on particular aspects of her projects. Other components of her career development include completing coursework through the University of North Texas’ School of Public Health, UTSW’s Pulmonary Vascular Biology Division, and UTSW’s Department of Clinical Sciences, including 25 hours of statistics, epidemiology, clinical research and vascular biology that are specifically tailored to meet her career development goals. Her proposal to take and complete coursework at the University of North Texas is noteworthy, since that institution is one of our CTSA partnering institutions. That partnership (which benefits both schools) has often involved seeing many of our young clinical research faculty take advanced courses in statistics and epidemiology at the University of North Texas, since these courses are (unfortunately) not available at UTSW. Finally and most importantly, will be immersed in an environment which was created and exists only to nourish the career development of clinical investigators. career development plan capitalizes on our existing multidisciplinary training environment, which includes formal didactic coursework, Socratic sessions, and mentoring in the conduct of Statements by Mentor, Co-Mentors, Consultants, Contributors Page 75 Principal Investigator/Program Director (Last, first, middle): Statements by Mentor, Co-Mentors, Consultants, Contributors Page 76 Principal Investigator/Program Director (Last, first, middle): critical, yet scarce. willingness to provide a fresh perspective on the pathogenesis of pulmonary hypertension is therefore laudable and very likely to lead to new pharmacologic approaches. In this proposal, also describes a training plan carefully built around the clinical research project. The project consists of two distinct approaches that address a plausible biological hypothesis from different angles. In this way, she will strengthen her mechanistic conclusions, since her hypothesis will be tested by both interventional human studies and epidemiological data; more importantly, she will strengthen her training to conduct both clinical trials and epidemiological studies. As you will see from the experimental plan, she has sharpened the focus of this proposal since the first submission, and has collected exciting preliminary data which supports her hypothesis. My dual role for this proposal is to provide both scientific and academic career advice. As a scientific advisor, my research background in vascular biology and specifically in signal transduction of the endothelium is appropriate. My work has focused on the molecular events which alter basic cell fate decisions such as proliferation and differentiation, relevant to the abnormal cellular pathology seen in pulmonary hypertension. I have served on journal editorial boards and grant review study sections relevant to vascular research, and am very familiar with research administration through my former position as Associate Chief of Staff for Research for the North Texas VA system. In addition, I am familiar with the design of research training programs as the Program Director of our Lung Biology and Disease T32 Training grant. This latter grant is a collaborative effort with Pediatric’s Pulmonary and Vascular Biology Division, providing a rich environment for to participate in and explore collaborative interactions with clinical and basic scientists. regularly presents her work at the research conferences associated with this program, Aside from these venues, I will dedicate at least 1-2 hours every month to meet with to discuss different scientific aspects of her project. Finally, as Division Chief, I will ensure that has at least 80% protected time for her research and that she continues to be placed in a fertile environment to foster her clinical research career. From a practical standpoint, we have recently recruited a third pulmonary hypertension clinical expert with 90% effort devoted to the clinical pulmonary hypertension program. Thus, the clinical programs in PH will continue to grow while allowing sufficient time to pursue her research. Through my additional service on the Institutional Promotions and Tenure Committee I am also able provide an objective assessment of progress towards academic promotion. In summary, I am very pleased to participate in K23 research project. is a truly outstanding candidate for this award and I expect great things from her as her career develops. Sincerely, Lance Terada, MD Professor of Internal Medicine and Chief, Division of Pulmonary and Critical Care Co-mentor: Lewis Rubin, MD: Dear Members of the Study Section, It is a distinct pleasure for me to write this letter providing my commitment to provide mentorship and my strongest support for the consideration of for a K23 Award. completed her training in Pulmonary and Critical Care Medicine at UCSD and obtained additional fellowship training in our Pulmonary Vascular Disease program while simultaneously taking several clinical research courses. This background has enabled her to return to Dallas with the skills and expertise to help build a pulmonary vascular clinical and patient-oriented investigative program at her alma mater, UT Southwestern. Indeed, hit the ground running, presenting and recently publishing novel work on cardiac imaging in patients with pulmonary arterial hypertension (PAH), which was generated since joining the faculty at the University of Texas. She now proposes to pursue investigation into an area of great importance both for our understanding of the pathogenesis of PAH and for its potential impact on public health. Specifically, reported previously that an association exists between methamphetamine use and pulmonary hypertension; she postulates that, as has been suggested in anorexigen-induced PAH, the serotonin signaling pathway may be responsible for this condition in particular and potentially in other forms of PAH. Using a two-pronged approach of clinical investigational and her epidemiologic training and skills, she will explore this hypothesis in greater detail. proposed work is highly innovative and of potential great significance in this field, where considerable progress has been made in developing treatments, but therapies targeting novel targets are sorely needed. has put together an outstanding group of mentors to help guide her career development at this critical stage, including individuals with expertise in cardiovascular clinical trial methodology (Dr. Packer), Vascular Biology and cell signaling (Dr. Terada), and Epidemiology / Statistics (Drs. Bernstein, Reich and Hailey). I am delighted to be part of this group, providing my support and guidance to specifically in the area of pulmonary vascular disease basic and patientoriented research. I will meet with her at least quarterly, usually at pre-arranged times coinciding with national scientific meetings (AHA, ATS, ACCP, ASCI), and will be available for teleconferences and email Statements by Mentor, Co-Mentors, Consultants, Contributors Page 77 Principal Investigator/Program Director (Last, first, middle): Statements by Mentor, Co-Mentors, Consultants, Contributors Page 78 Principal Investigator/Program Director (Last, first, middle): Mentoring Record: Milton Packer, MD David Markham, MD (junior faculty) Russell Canham, MD (fellow) Paolo Columbo, MD (junior faculty) Rachel Bijou, MD (post-doc) Allen Anderson, MD (junior faculty) Ulle Jorde, MD (junior faculty) Matthew Maurer, MD (fellow-jr faculty) Deborah Ascheim, MD (post-doc) Daniel Burkhoff, MD PhD (jr faculty) Stuart Katz, MD (junior faculty) Jon. Sackner-Bernstein, MD (post-doc) Henry Krum, PhD (post-doc) Jill Kalman, MD (post-doc) Paul Hauptman, MD (post-doc) Gerald Neuberg, MD (post-doc) David Pinsky, MD (post-doc) Lawrence Baruch, MD (post-doc) Marrick Kurkin, MD (post-doc) Stephen Gottlieb, MD (post-doc) 06-C 04-06 02-04 00-03 00-02 99-05 98-05 97-05 94-00 93-00 92-96 91-95 90-92 90-91 89-91 88-89 87-89 86-92 85-90 Project Proteomic signatures in CHF Genetic epidemiology of CHF Endothelial dysfunction in CHF Brain naturetic peptide in CHF Device therapy in heart failure Aldosterone in heart failure Heart failure with preserved EF Device therapy for heart failure Cardiac dynamics in heart failure Endothelial dysfunction in CHF CHF: efficacy of beta-blockers CHF: efficacy of beta-blockers Congestive Heart Failure QALI and outcomes in advanced CHF CHF: efficacy of beta-blockers Vascular phenotype modulation Cholesterol mgmt and echo CHF: neurohormonal antag. Novel treatments of CHF Current or last known position Asst Prof, UTSW Asst Prof, Wash. U. Asst Prof, Columbia P&S, PI NIH R29 Asst Prof, Columbia P&S Assoc Prof, Univ of Chicago Assoc Prof, NYU Dir, CHF Prog, PI NIH K23 / R01 Assoc Prof, Columbia P&S, PI NIH R01 Assoc Prof, Mt. Sinai, PI NIH U01 Assoc Prof, Columbia P&S, PI NIH U01 Prof, Medicine Yale Univ, PI NIH R01 Assoc Director, FDA Center for Devices & … Prof, Monash U, Melbourne, MRC funding Assoc Prof, NYC University Prof, St. Louis University Assoc Prof, Columbia P&S Chief & Prof, Univ of Michigan, 3 R01s, PI of T32 Asst Prof, Mount Sinai School of Medicine Prof Clin Med, Columbia P&S Prof Medicine, U Maryland RESEARCH TRAINEES: Lance Terada, MD, Plus 3 Others Current Position Rhonda F. Souza, MD 99-04 Associate Prof. Medicine, UTSW Ying Gu, MD 99-06 Medical Extern, New York You Cheng Xu, PhD 99-06 Research Associate, UTSW Fiemu E. Nwariaku, MD 00-05 Associate Prof. Surgery, UTSW Ru Feng Wu, MD, PhD 01-C Instructor, UTSW George A. Sarosi, Jr., MD 03-08 Assistant Prof. Surgery, Univ. Florida Shuqi Zhang, MD 04-06 Post-doc, UTSW Amit Verma, MD 04-05 Assistant Prof. of Medicine, NYU Zhenyi Ma, PhD 04-C Instructor, UTSW Subhash Banerjee, MD 05-08 Assistant Prof. Medicine, UTSW Simrit Parmar, MD 05-09 Assistant Prof. Medicine, MD Anderson Zhe Liu, PhD 08-C Research Associate, UTSW Yimei Gong, MD Matthew Leveno, MD Rosechelle Ruggiero, MD Pritam Ghosh, MD Guosheng Fu, PhD 09-C 10-C 10-C 10-C 10-C Research Associate, UTSW Research Fellow, UTSW Assistant Professor, UTSW Research Fellow, UTSW Postoctoral Fellow, UTSW CLINICAL AND RESEARCH TRAINEES: Lewis J. Rubin, MD, Plus 21 others Current Position Yuan, JXJ (Postdoc), MD PhD 88-91 Prof of Medicine and Assoc Director for Research Training, UCSD Amelung, P (Postdoc), MD 90-93 Clinical Assoc. Professor of Medicine, U of Maryland, Baltimore Thurm, C (Postdoc), MD 90-93 Chief, Pulmonary Medicine, Jamaica Hospital New York Costa, J (Postdoc), MD 93-97 Clinical Assistant Prof of Medicine, U of Maryland, Baltimore Moore, W (Postdoc), MD 94-97 Assoc Prof, Bowman Gray Sch of Med, Winston Salem, NC Mpe, MJ (Postdoc), MD 96-97 Dept of Intensive Care, University of Limpopo, South Africa Buck, T (Postdoc), MD 95-98 Annapolis Medical Service, Annapolis, MD Soriano, C (Postdoc), MD 97-99 Associate Physician, PCCM, Greater Baltimore Med Center; Assist Director Test, V (Postdoc), MD 2001 Assoc Clinical Professor of Medicine, Clinical Service Chief, UCSD Kim, H “Nick” (Postdoc), MD 98-01 Associate Professor, Fellowship Program Director, UCSD Bailey, C (Postdoc), MD 99-02 Associate Professor, Harvard Medical School Helmersen, D (Postdoc), MD 2002 Clinical Assist Prof of Medicine, Peter Lougheed Centre, Calgary, Canada Morales Blanhir, J (Postdoc), MD 02-03 Attending Physician, Nat Inst of Resp Disease, Mexico City, Mexico Zagolin Blancaire, M (Postdoc), MD 2003 Attending Pulmonologist, Nat. Institute of Thorax, Santiago, Chile Williamson, T (Postdoc), MD 02-03 Med Director, PHTN Program, U of Kansas; Assoc Professor Kayikcioglu, M (Postdoc), MD 2005 Cardiology Faculty, Ege U. School of Medicine, Bornova, Turkey Statements by Mentor, Co-Mentors, Consultants, Contributors Page 79 Principal Investigator/Program Director (Last, first, middle): The Institution: The University of Texas Southwestern Medical Center (UTSW) ranks among the top academic medical centers in the world. It is situated northwest of downtown Dallas, on a 231 acre campus, encompassing over 22 buildings with more than 7.4 million square feet of space. The excellence of the UT Southwestern faculty is well recognized as it includes 4 active Nobel Laureates, 18 members of the National Academy of Sciences, 18 member of the Institute of Medicine, and 13 members of the American Academy of Arts and Sciences. UTSW trains more than 4,000 physicians, medical scientists, and allied health-care professionals each year through three degree-granting institutions: Southwestern Medical School, Southwestern Graduate School of Biomedical Sciences, and Southwestern Allied Health Sciences School. Over 3,500 research projects are under way with more than $400 million in annual funding, conducted within more than 582,000 square feet of research space, with more under construction. The Department of Clinical Sciences and KL2 Clinical Scholars Programs With the establishment of the Department of Clinical Science, UT Southwestern Medical Center is fast expanding its prominence as a center for clinical and translational research. This includes a faculty that is internationally renowned with highly productive investigators across all fields of clinical and basic science study, a large clinically and ethnically diverse patient base located in the immediate proximity, and more than $150 million of peer-reviewed grant support for clinical and translational studies. The comprehensive and highly successful K30-supported curriculum specifically targeted to clinical scientists combined with the training and career development program supported by a NIH Clinical and Translational Science Award (CTSA) KL2 mechanism provide a superb infrastructure to facilitate novel clinical research – and to provide a clinical research home for trainees and recent graduates. Benefits include weekly clinical research and work in progress meetings, monthly career development seminars, and resources to assist in the completion of clinical research. This structured, interactive, highly disciplined environment makes UT Southwestern an ideal training environment. Additionally, advanced training in statistics, epidemiology and other disciplines is available through partner institutions in the CTSA, including the University of North Texas’ School of Public Health. UT Southwestern provides a strongly supportive environment for the development of young clinical investigators, and the Clinical Science Department facilitates this by supporting high quality clinical research and by focusing on the training and career development of clinical investigators. Core functions of the Department of Clinical Science relevant to research plan include the availability of experts in biostatistics, biomedical Informatics, epidemiology and pharmacology. Several of these experts are listed as advisors in her training plan, but the wealth of expertise across many other disciplines also serves as an invaluable resource for “as needed” advice. The Pulmonary Vascular Biology Division UT Southwestern is the only institution in the country to support a separate division devoted entirely to pulmonary vascular research. The Pulmonary and Vascular Biology Division provides a valuable resource for campus-wide investigative endeavors. This is represented by active collaborations between Pulmonary and Vascular Biology faculty and other UT Southwestern faculty in the departments of Internal Medicine, Cell Biology, Physiology and Pharmacology, and by participation of Pulmonary and Vascular Biology faculty in numerous training and center grants across the campus. The division’s mission is to expand basic understanding of lung and vascular diseases, striving to gain new knowledge that will ultimately lead to new diagnostic, prophylactic and therapeutic strategies. This includes an on-going multi-disciplinary pulmonary vascular biology work-in-progress seminar, held twice monthly in collaboration with several other divisions, and specific coursework focused on pulmonary vascular biology advances, offered on an annual basis. Institutional Environment Page 80 Principal Investigator/Program Director (Last, first, middle): Institutional Commitment to Career Development Page 81 Principal Investigator/Program Director (Last, first, middle): SPECIFIC AIMS: Pulmonary arterial hypertension (PAH) is a progressive, life-threatening disorder of unknown cause. Serotonin (5HT) is thought to contribute to the pathogenesis of PAH, but clinical studies are few and uninformative. Older studies conducted more than a decade ago focused on the 5HT 2A receptor, subsequently shown to be less important in human lungs compared with the lungs of rats and mice. Other studies have focused on circulating 5HT levels, but free 5HT levels do not accurately represent tissue exposure. Recent progress in the molecular pharmacology of 5HT signaling has allowed us to rethink its role in the pulmonary circulation. We hypothesize that the 5HT transporter is a key contributor to pulmonary hypertension. Serotonin uptake by the transporter has been shown to promote pulmonary artery (PA) smooth muscle cell growth, and overexpression of the transporter is sufficient to cause PH in animals. Additionally, although 5HT receptor signaling promotes acute vasoconstriction through at least three receptors, inhibitors of the 5HT transporter (selective 5HT reuptake inhibitors, SSRIs) reduce pulmonary vascular remodeling and long-term pulmonary hypertension severity in animals more effectively than 5HT receptor antagonists. Little is known about the effects of this class of drugs in patients with PAH. Accordingly, our experimental hypothesis is that uptake of 5HT or a 5HT transporter substrate by the 5HT transporter can promote PAH in humans, such that: • Blocking the 5HT transporter with fluoxetine, an SSRI, will reduce pulmonary vascular resistance (PVR) in PAH. SSRIs reduce the growth promoting effects of 5HT in vitro and reduce pulmonary hypertension severity (including vascular remodeling) in animal models of PAH. Additionally, SSRIs were well tolerated in a pilot study (N=6, 5 completed) that we conducted, with preliminary evidence suggesting a hemodynamic benefit. Further study in a larger number of patients is needed. • Use of 5HT transporter substrates (as a class) will be associated with an increased risk of idiopathic PAH. Fenfluramine and aminorex are 5HT transporter substrates and diet-pills that were found to be strongly associated with idiopathic PAH in prior population incidence studies and casecontrol studies. Subsequently, we noted that methamphetamine, also a 5HT transporter substrate, appeared to be strongly associated with idiopathic PAH. We therefore plan to evaluate the potential association of use of methamphetamine and other 5HT pathway medications with PAH in a case-control study. Aim 1: To determine whether fluoxetine lowers pulmonary vascular resistance in patients with pulmonary arterial hypertension by conducting a randomized double-blind placebo-controlled trial Primary Hypothesis: Fluoxetine treatment for 24 weeks will lead to significantly lower pulmonary vascular resistance compared with placebo treatment in 34 patients with PAH in patients treated in a blinded clinical trial. Study: In this randomized, double blind placebo controlled clinical trial, 34 patients with PAH will be randomized to placebo vs. fluoxetine for 24 weeks. Catheterization will be performed at baseline and 24 weeks. Change in PVR will be the primary endpoint; other hemodynamic endpoints, quality of life, QIDS-SR depression scale, functional class and six-minute walk distance will also be evaluated. Aim 2: To determine whether medications that increase 5HT transporter activity are associated with an increased risk of PAH by carrying out a case-control study Primary hypotheses: Patients with idiopathic PAH will report significantly higher rates of use of the 5HT transporter substrates methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) when compared with controls. Study: In this case-control study, prior use of medications with 5HT signaling activity will be queried by in-person interview in 70 idiopathic PAH cases, 70 other PH controls and 70 normal controls, with results adjusted for age, gender and race. Medications of interest will include use of methamphetamine or MDMA (5HT transporter substrates, primary endpoint); use of stimulants without significant 5HT activity (active control) and as exploratory endpoints, use of SSRIs, cocaine, and antimigraine medications (5HT receptor agonists). Significance: Despite the identification of numerous signaling pathways that may contribute to the development of PAH, the role of 5HT in the clinical setting remains unconfirmed. This study will help to establish whether the 5HT transporter is a key contributor to pulmonary hypertension. We are uniquely qualified to complete this work, as shown by our prior studies in both areas. Specific Aims Page 82 Principal Investigator/Program Director (Last, first, middle): The Research Plan is Divided into a Part I (Clinical Trial) and a Part II (Case-Control Study) Significance (Parts I and II): Pulmonary arterial hypertension (PAH) is a life threatening pulmonary vascular condition that often affects young, otherwise healthy patients. Mortality is 50% at five years, even with current therapy1-2. Serotonin (5HT) has long been considered a potential contributor to PAH, but enthusiasm for clinical studies waned after antagonists to the serotonin 2A receptor failed to significantly improve pulmonary hemodynamics. However, recent progress in the molecular pharmacology of 5HT signaling has allowed us to rethink its role in the pulmonary circulation, and we propose that the 5HT transporter plays an important role in the pathophysiology of PAH. Specifically, we hypothesize that uptake of either 5HT or a 5HT transporter substrate can promote PAH, while blocking uptake of 5HT with a selective serotonin reuptake inhibitor (SSRI) will reduce the severity of PAH. This hypothesis is supported by animal studies: long-term exposure to fenfluramine, a 5HT transporter substrate, causes PH, while SSRIs prevent and reverse PH3-5. We plan two studies testing these hypotheses: (1) A case-control study evaluating whether 5HT transporter substrates are associated with idiopathic PAH, and (2) A randomized double-blind placebo controlled study to determine whether blocking 5HT uptake with fluoxetine lowers pulmonary vascular resistance (PVR) in PAH. These studies, if successful, could pave the way toward novel therapies. PART I: CLINICAL TRIAL OF FLUOXETINE IN PAH I.A Introduction: We propose that the 5HT transporter is a key contributor to PAH: • Animals that over-express the 5HT transporter spontaneously develop PH6-7. • Human subjects with idiopathic PAH (IPAH) over-express the 5HT transporter on all cells studied including platelets and pulmonary arterial smooth muscle cells (PASMC) 8. • Uptake of 5HT by the transporter promotes PASMC growth in vitro. Specifically, growth is promoted by serotonylation of intracellular proteins and the generation of reactive oxygen species9-10. • PASMC growth is markedly reduced by blocking the 5HT transporter with an SSRI8,11. Table 1: Serotonin Receptor and Transporter Antagonists in Animals and in Human Subjects Hypoxic PH Monocrotaline PH Human data: 1B receptor Mixed results – at best No studies Triptans (agonists) increase PAP 25% in normals16; no Antagonist moderately effective4,12-13 studies in PAH – thus use will be queried in Aim 2 2A receptor Failed to prevent hypoxic Prevented but did not Ketanserin lowered PVR moderately but caused Antagonist PH4 reverse monocrotaline PH15 hypotension, and long-term studies were not done17-18 2B receptor Prevented hypoxic PH14 No studies of isolated 2B Abstract only – antagonist improved exertional PVR in antagonist antagonism lung disease PH19 Transporter Prevented hypoxic PH4 Prevented and reversed SSRIs were associated with improved survival in antag. (SSRI) monocrotaline PH5 observational studies in PAH (p<0.05 in one)20-21 Table: SSRIs are effective in monocrotaline and hypoxic PH, are able to both prevent and reverse monocrotaline PH, and are more effective than 5HT 2A and 1B receptor blockers in head to head studies4. We propose that blocking the 5HT transporter with six-months treatment with fluoxetine will lower PVR and lead to improved symptoms and exercise capacity in patients with PAH. • SSRIs lowered pulmonary arterial (PA) pressure and reduce vascular remodeling in hypoxic and monocrotaline models of PH4-5. • SSRI use may be associated with longer survival in PAH, though epidemiology data is limited20-21 I.B Innovation: Three classes of medications are approved for the treatment of PAH22. However, survival remains poor, even with the use of continuous intravenous epoprostenol, thought to be the most potent of the available therapies. There is an urgent need to identify alternative therapeutic pathways1. Despite this, most patients who enroll in clinical trials today will receive a “me-too” drug that belongs to one of the existing commercially available medication classes (per clinical trials.gov23), such as an ‘improved’ endothelin antagonist or a 'novel' phosphodiesterase-type 5 inhibitor (PDE-5 inhibitor). We propose to carry out a phase 2 clinical trial involving a truly novel signaling pathway that is supported by strong data in animal models and encouraging preliminary data in patients with PAH. If successful, we anticipate the need for larger clinical trials of 5HT signaling modulation in PAH. I.C Approach: This study aims to evaluate the effects of 5HT transporter blockade with fluoxetine in a 6-month hemodynamics-based double-blind placebo controlled clinical trial that will enroll 34 patients with PAH. The primary endpoint is the change in pulmonary vascular resistance (PVR). This variable was selected because changes in PVR with therapy predict mortality in PAH and because it has long been used as an “intermediate” Research Strategy Page 83 Principal Investigator/Program Director (Last, first, middle): predictor of later success in PAH trials. Patients will undergo hemodynamic evaluation before and after treatment, and clinical outcomes (exercise capacity and a SF-36 quality of life survey) will be assessed. I.D Preliminary Data: Experience in clinical trials: I have served as the site-principal investigator on 7 multi-center clinical trials and as a steering committee member on two clinical trials (EPITOME and GRIPHON, Actelion). My role has included contributions to study design, data analysis and manuscripts. These studies demonstrate my experience in conducting trials, managing budgets, meeting enrollment goals and following IRB regulations. Pilot Study: In my original K23 application, I proposed a pilot study involving open-label use of two 5HTtargeting medications: reserpine, a 5HT depleting agent, and fluoxetine, an SSRI, to be given alone for 12 weeks and then in combination for 12 weeks. My original hypothesis was that the use of fluoxetine to block the 5HT transporter combined with reserpine to deplete stored pulmonary and platelet 5HT (and reduce 5HT receptor signaling) would have a highly favorable effect on PVR. Although the K23 application was not funded, I nevertheless proceeded to carry out the study. Funding was obtained through a competitive intramural grant through the North and Central Texas Clinical and Translational Science Initiative (NCTCTSI). NCTCTSI grants provide research support only and fewer than 2-3 grants are funded at the highest dollar amount ($50,000) for which I applied. The reserpine arm of the study began first, with three patients receiving open label reserpine for 12 weeks. All three patients reported increased dyspnea, lower extremity edema and / or had elevated JVD during weeks 2-8. One patient withdrew from the study, while symptoms gradually improved in the other two patients. Hemodynamic evaluation at 12 weeks in those two patients showed a marked (40%) decline in PVR in one patient and a minor change in PVR (3% decline) in the other. The discordance between early worsening symptoms and late improvement in symptoms and hemodynamics was reviewed carefully with an independent study safety physician, with our local IRB, and with my mentors, and two modifications were made: fluoxetine would be given first and the dose of reserpine would be decreased 50% in the hopes that this would improve tolerability. The next two patients tolerated fluoxetine for 12 weeks without difficulty, but both developed dyspnea within four weeks of starting reserpine, and one patient had exertional syncope. The cause for this early worsening remains unclear; however, patients with left-sided heart failure have been reported to experience worsening heart failure following initiation of reserpine, attributed to catecholamine depletion. Therefore, despite the meaningful long-term improvement at 12 weeks in one patient, we concluded that the risks outweighed any potential for benefit and we elected to discontinue the reserpine arm of the study. An additional four patients then received fluoxetine (only) for 12 weeks, bringing the total to six patients who received fluoxetine alone (no reserpine) on top of background PAH therapy. Fluoxetine was begun at 20 mg and increased to 40 mg at two weeks and 80 mg at four weeks No serious adverse events were seen, but anxiety and trouble sleeping caused one patient to withdraw during the second week and caused two patients to undergo dose reductions to 20-40 mg. Hemodynamic results are shown in the figures; PVR declined 16% (p=NS) and CI improved 19% (p<0.05) with no significant change in PA mean. Three of five patients completing the study reported improved symptoms, but a formal symptom scale was not utilized. The QIDSSR depression scale also improved by >50% in two patients (50% is considered significant) despite the absence of reported depression at study entry – but it is unclear whether this was from treatment of unrecognized depression or improved overall status. Overall interpretation and modifications: this open label pilot study suggests a possible beneficial effect based on the change in PVR and CI which, if seen in a larger study, would be in-line with other PAH therapies. This preliminary experience led to four changes in the current proposal: first, the dose titration will be slowed such that patients will not be given the full dose until week 12 in the hope that this may improve tolerability of the higher doses. Second, Research Strategy Page 84 Principal Investigator/Program Director (Last, first, middle): although a dose response effect was not evident in our five patients, dose does not necessarily reflect blood levels, and we will therefore check fluoxetine blood levels at the time of follow-up catheterization to more thoroughly assess this. Third, the study duration will be increased to 6 months based on review of recent addon therapy studies in PAH (longer duration may be needed vs. de novo therapy studies). Fourth, we will add two quality of life measures to better detect overall change in clinical status, in addition to continued use of a depression scale. Clinical Trial: Research Design and Methods: I.E Study Subjects: Patients with PAH and persistently elevated PVR after treatment with one or more approved therapies will be eligible, including endothelin-1 receptor antagonists (ambrisentan or bosentan), phosphodiesterase-5 inhibitors (sildenafil or tadalafil) and prostacyclins (epoprostenol, treprostinil, iloprost). Table 2: Inclusion / Exclusion Criteria Inclusion 1. WHO Group I PAH subtypes of idiopathic PAH, drugs / toxins, or connective tissue disease associated PAH, diagnosed by complete work-up (VQ or CTA, echo, labs, PFTs, catheterization)* 2. Age 16-80 3. WHO Functional Class II or III (definition in “procedures” below) 4. Catheterization within 2 weeks of study entry with mPAP ≥ 25 mmHg, wedge ≤ 15 mmHg, and PVR ≥ 5 Wood units**. 5. Contraception use, (-) urine pregnancy test, not breast feeding (women of childbearing potential)† 6. One or more approved PAH therapies for ≥3 months, no change in dose for 1 month (endothelin-1 antagonist, phosphodiesterase5 inhibitor, prostacyclin / prostacyclin analog). Novel approved therapies in one of the three existing classes will also be acceptable as background therapy if they become available during the course of the study; other medication classes are excluded Exclusion 1. WHO Functional Class IV or listed for lung transplant (Reason: may be too ill / unstable) 2. Other cause for pulmonary hypertension: all other WHO group I diseases (including but not limited to congenital heart disease, liver disease, HIV), and WHO Groups II-V (i.e. left heart disease, lung disease, chronic PE and miscellaneous causes)24. a. FEV1/FVC < 70% and FEV1 < 60% predicted, or TLC < 60% predicted unless no more than mild lung disease clinically and by computed tomography imaging b. High probability VQ or positive CTA c. Left ventricular ejection fraction <40% 3. Depression 4. Severe liver, renal or other medical or physical disease preventing completion of the study procedures 5. Use of antidepressants within 3 months *ACCP consensus conference definitions; these sub-types in general respond similarly to PAH therapies25. **Higher PVR may increase chance of benefit in PAH in general, based on other studies. †Contraceptive pill, implant, vaginal ring, IUD, abstinent, or vasectomised partner. I.F Study Methods: Medication: 34 patients will be randomized to fluoxetine vs. placebo beginning with 20 mg daily and increasing to 40 mg at four weeks, 60 mg at 8 weeks, and 80 mg at 12 weeks. Efficacy assessments will be completed at 24 weeks. Procedures: caths -- I will perform right heart catheterizations in the St. Paul cath laboratory using the internal jugular or femoral veins for venous access. Procedures will be conducted per cath-lab usual routine, including standard safety monitoring (EKG, oximetry, blood pressure). Ultrasound and fluoroscopy will be used in catheter placement. Measures will include end-expiratory right atrial (RA), PA and wedge, systemic noninvasive blood pressure, heart rate, cardiac output (CO, by thermodilution, performed in triplicate and averaged), pulmonary (SvO2) oxygen saturation, systemic pulse-ox oxygen saturation, hemoglobin. Calculations will include PVR and Fick CO (using estimated VO2 from a LaFarge table)26. Wedge measurement will be measured with extra caution including careful review of the waveform and if needed partial deflation of the balloon, repositioning of the catheter, and confirmation of position by oxygen saturation, if uncertainty remains. If difficulty persists, left ventricular end diastolic pressure will be obtained and substituted for the wedge pressure. Other procedures: Six minute walk distance (6MWD) is the distance a patient can walk in 6 minutes using a long (≥50 meters) hallway. 6MWD correlates with VO2 max, predicts survival in PAH, and is an important efficacy endpoint in pivotal PAH clinical trials. WHO functional class is a symptom scale with 4 classes: no symptoms (class I) or symptoms with ordinary activity (II), less than ordinary activity (III) or at rest (IV). QIDS-SR depression questionnaire is a validated 16 question depression scale that is completed by patient self-report27. CAMPHOR and the SF-36 are quality of life scales (CAMPHOR is PH specific)28. Labs: All patients will undergo p-selectin, soluble CD 40L, betathromboglobulin, 24-hour urine 5-HIAA and fluoxetine level at baseline and week 24 (table 3; see also “substudies” in tables 4-5). Research Strategy Page 85 Principal Investigator/Program Director (Last, first, middle): Primary Outcome Variables: Change in PVR, calculated as [(PA mean – wedge) / Fick CO] will be the primary endpoint. PVR was chosen because it is a sensitive measure of improved hemodynamics in PAH, and improvement in PVR has also been strongly associated with improved exercise capacity and survival. PVR has also improved with all of the current FDA-approved PAH therapies. Fick CO was chosen over thermodilution because Fick appears to have greater precision (but not accuracy); since we are most interested in change in PVR, then use of the Fick CO will improve power by minimizing random variability. Screening, enrollment, follow-up: During screening, inclusion/exclusion criteria will be reviewed and if met, informed consent will be obtained. Patients will be seen by the MD and coordinator at each visit, with tests as indicated in the chart. All visits and study procedures should be within +/- 7 days. Table 3: Study Visits for Randomized Placebo Controlled Trial of Fluoxetine Baseline Week 4 Week 8 Week 12 Week 18 Week 24 Clinic visit / vitals / exam X X X X X X 6MWD, WHO Class X X X X X X QIDS-SR X X X CAMPHOR. SF-36 X X X 24-hr Urine / blood-work X X Catheterization X X Fluoxetine (mg) Start 20 mg 40 mg 60 mg 80 mg 80 mg 80 mg Table: Medication doses increase every 4 weeks, as tolerated. Pregnancy testing (when appropriate) will also be done at entry and prior to caths. 6MWD: six minute walk distance. WHO: World Health Organization. QIDS-SR: Depression Scale. Early withdrawal: Patients exiting the study early for any reason will complete the week 24 procedures, whenever possible. This includes those who request study discontinuation (for side effects or other reasons) and those who worsen during the study and require additional medications. Blinding will be maintained unless the treating clinician feels the patient’s safety may be compromised. Discontinuation will ideally be gradual, to be accomplished when possible over up to 4 weeks. Routine end of study: patients will be offered treatment with open-label fluoxetine. Transition will be via blinded titration and once on completely open label therapy, patients will exit the study except for planned chart review after 12 months to obtain hemodynamic results, if available (with patient consent for chart review; most patients undergo a routine annual catheterization, per center routine). Safety concerns: There has previously been concern that SSRIs could worsen PH by increasing 5HT receptor signaling. However, when 5HT levels are measured carefully (microdialysis techniques), circulating free 5HT levels increase only modestly and normalize within 14 days29. Clinical safety data is also generally reassuring: there are no case reports of worsening PAH with SSRI use, even though ~20% of patients take SSRIs20-21. Furthermore, clinical trials in patients with coronary artery disease or heart failure have found no concerning safety signals for SSRIs. Cohort studies in PAH also suggest that users of SSRI have a survival as good or better than the survival of nonusers of SSRI therapy (HR 0.35 (0.14-0.88)20 and 0.53 (0.07-3.9)21. We will monitor patients closely with visits every 4-6 weeks, as above. Patients will be observed closely for expected SSRI side effects (gastrointestinal symptoms, anxiety and other psychiatric symptoms) and for worsening PH. No patients with active depression will be enrolled, and patients who develop signs of depression will be withdrawn and undergo psychiatric evaluation. A local independent data-safety monitoring board will review all AEs and SAEs and will review the overall study every 6 months. Substudies: 5HIAA and Platelet / Endothelial Activation in PAH Before and After Fluoxetine: Table 4: 5HIAA Sub-Study: Does Fluoxetine Alter Serotonin Production? Aim A: To determine whether baseline 5HIAA levels predict response to fluoxetine. 5HT production and turnover appear to be increased in PAH: PAH endothelial cells make more 5HT, PAH neuroendocrine cells and platelets store more 5HT, and 5HIAA, the primary 5HT metabolite, was seen at increased levels in two small PAH case series30-37. We hypothesize that 5HIAA (1) Will be elevated in PAH vs. controls, (2) Will correlate with baseline PVR, and (3) Will correlate inversely with change in PVR after 24 weeks of fluoxetine. In other words, we propose that patients with the highest 5HIAA levels will have more severe disease and have a better response to fluoxetine. Aim B : To determine whether fluoxetine leads to a reduction in 5HIAA levels. Most 5HT is metabolized to 5-HIAA, and 5HIAA levels correlate with total body 5HT production. We hypothesize that fluoxetine lowers 5HT production and turnover systemically, as measured by 5HIAA levels. Although SSRIs block the 5HT transporter, circulating 5HT levels normalize over the long-run29. It is unclear whether this occurs via alternative clearance mechanisms or altered production*. Study: 34 clinical trial patients and 16 controls (unrelated family members) will undergo 24-hour urine Research Strategy Page 86 Principal Investigator/Program Director (Last, first, middle): 5HIAA, tested in the clinical laboratory. Trial participants will undergo a repeat test at week 24. Comparisons will be (A1) 5HIAA level: IPAH vs. control. (A2) Correlations: baseline 5HIAA vs. baseline PVR and vs. change in PVR at 24 weeks, IPAH patients. (B) Change in 5HIAA level: baseline vs. week 24, IPAH patients. *CNS studies exist and fluoxetine does lower 5HT production in some regions - but central 5HT production is via a different gene. Table-5: Platelet Sub-Study: Does Fluoxetine Alter Platelet Activation? Aim C: To determine whether fluoxetine reduces markers of platelets and endothelial activation in PAH. Platelet and endothelial cell activation is seen in PAH, may correlate with PAH disease severity, and can fall with treatment of PAH38-42. We hypothesize that platelet activation will fall with SSRI therapy, because SSRIs reduce platelet activation in other settings and because SSRIs deplete platelet 5HT43-44. However, platelet aggregation tests have been inconsistent in PAH40. We therefore plan to measure indirect markers of platelet and endothelial cell activation including soluble p-selectin, soluble CD40 ligand and beta-thromboglobulin. Prior studies suggest these markers are elevated in PAH, and they are readily measured38,45. Evaluations will be performed at baseline and week 24 in all patients in the clinical trial and at baseline in 16 controls. Because this will be exploratory, we will also bank blood so that if we obtain significant findings, future study can be considered. Elisa kits for the three tests will be obtained from R&D Systems, Minneapolis, MN, USA. Assays will be performed in Dr. Terada’s lab. Comparisons for each measure will be (1) Idiopathic PAH vs. CTD-PAH vs. Control, and (2) Change in level at 24 weeks, fluoxetine vs. placebo. I.G Statistical Analysis Aim 1: To determine whether fluoxetine lowers pulmonary vascular resistance in patients with pulmonary arterial hypertension by conducting a randomized double-blind placebo-controlled trial 1. Study design and measurements: 34 patients will be randomized 1:1 to fluoxetine vs. placebo for 12 weeks. Patients will undergo cath, 6MWD testing, QIDS-SR depression scale testing, SF-36, CAMPHOR quality of life and functional class assessment pre-treatment and after 24 weeks. 2. Efficacy endpoints: Primary endpoint: Change in PVR between baseline and end of study (treatment vs. placebo). Secondary endpoints include change in: RA, PA and wedge pressure, CO, SvO2, Camphor, QIDS-SR, SF-36, systemic BP and HR, functional class and change in 6MWD (all treatment vs. placebo). Safety endpoints: will be assessed by tabulating adverse events. Open label hemodynamics after one year will also be presented descriptively and vs. baseline. 3. Comparisons and evaluation of the data: All statistical testing will be between group comparisons evaluating the change between baseline and 24 week follow-up. Student’s t-tests will be used for significance testing if the data are normally distributed with no missing data. If data are missing due to dropouts or lack of study completion, patients with worsening symptoms will be assigned worst rank, and a Wilcoxon rank sum test will be used. Decisions about the handling of missing data will be made prior to unblinding. The Wilcoxon rank sum test will also be used to evaluate the ordinal results from the QIDS-SR, the SF-36, and changes in WHO functional class. 4. Assumptions and anticipated magnitude of treatment effect and power: Baseline PVR is estimated to be 9 wood units, based on preliminary data PVR of 8.4 wood units + added inclusion requirement of PVR ≥5 wood units. Effect size: this study will be powered to detect a 20% improvement in fluoxetine patients + a 5% worsening in the placebo patients with 30 of 34 patients undergoing an end of study catheterization. In earlier studies, approved PAH therapies (endothelin-1 antagonists, PDE-5 inhibitors and prostacyclins) have lowered PVR 12-39% (all except one: 20-39%), and treatment with placebo is typically accompanied by worsening of 5% or more. Standard deviation (SD): we anticipate a SD 28% of baseline PVR for fluoxetine and 20% of baseline for placebo patients. This estimate is based on clinical trials in PH and is conservative (actual SD likely to be lower); in our fluoxetine pilot study the SD for the active treatment patients was 24% of baseline PVR. Summary: With 30 patients completing, this study will have 80% power (α=0.05) to detect a between-group difference assuming 20% improvement with fluoxetine and 5% worsening with placebo with a SD of 28% and 20% in each group, respectively. Note that enrollment will be increased, if needed, to achieve 30 completions. (References for SD and other assumptions:46-52) Safety and tolerability: An important secondary endpoint will be safety and dosing tolerability. This will be evaluated both qualitatively and quantitatively. We anticipate that ≥80% of patients will be able to complete the study (completing either 24 weeks or undergoing early end of study) and ≥70% of enrolled patients will complete it without without suffering a serious adverse event judged as possibly or likely related to medication (SAE; death, hospitalization, risk to life or permanent disability). The determination of “likely” related is to be made by an independent physician who will review the data for all SAEs. These modest goals are chosen because all patients will be seriously ill. Research Strategy Page 87 Principal Investigator/Program Director (Last, first, middle): Potential Problems: Enrollment: If enrollment is slower than expected, the patient population will be expanded to include patients with repaired, simple congenital heart disease and patients with HIV; these patients often respond similarly to medications. However, we enroll approximately 40 patients per year in clinical trials from a patient population that includes ~60 new PAH patients yearly and more than 300 active PAH patients and we do not anticipate difficulty in enrolling 34 patients over 3-4 years. Completion: we need 30 patients to undergo a post-treatment catheterization and other week 24 assessments out of a planned enrollment of 34 patients. This allows for 4 (12.5%) patients to drop out without a post-Rx cath and still have sufficient power. Based on past experience, this should be achievable: even patients leaving early typically allow end of study assessments. If greater numbers dropout, then we will increase enrollment with funding from other sources (matching institutional resources; see letter from Dr. Packer). Smaller degree of improvement than expected: this is certainly possible but will not be known until study completion; if seen, we will look closely at individual patients to determine whether future studies are indicated and whether (1) The primary issue is likely to be sample size; -or- (2) A disease sub-group or other subgroup has more positive results (i.e. only patients with idiopathic PAH, or only patients on endothelin-1 antagonists, or only patients with high 5HIAA levels). If any of the above are seen, additional study of that particular sub-group will be required and will be the subject of future grant applications. Safety: because these patients are sick at baseline, adverse events from the disease are not unexpected. We will regularly review adverse events (AE; blinded) in order to identify any concerns, and a data safety monitory committee will review the AE and SAE data prior to each IRB continuing review (every six months) including access to unblinded data if needed. PART B: ARE SEROTONIN TRANSPORTER SUBSTRATES ASSOCIATED WITH IDIOPATHIC PAH? II.A Introduction: Epidemiologic studies have found strong associations between idiopathic PAH and the use of diet-pills, reported in 16-32% of patients53-58. Although the fenfluramines were most strongly implicated, use of amphetamine-containing diet-pills was also increased (p=NS). Based on these results, I completed a casecontrol study of stimulant use at UCSD (methamphetamine, amphetamine and cocaine), and found that prior use was strongly associated with IPAH60. Additional study is important because it remains unclear which stimulants are associated with idiopathic PAH. Furthermore, widespread exposure to stimulants is common: prescription stimulants are taken by >5% of children for attention deficit hyperactivity disorder (ADHD), 8-16% of adults report illicit cocaine or methamphetamine use, and more than 7% report prior use of prescriptiondiversion use of methylphenidate or amphetamine. Our hypothesis is that use of MDMA and methamphetamine (5HT transporter substrates) will be associated with idiopathic PAH, while use of the non-5HT transporter substrate stimulants will not be associated with idiopathic PAH. Table 6: Associations Between Medications and Idiopathic PAH, with Mechanisms of Action Association with Idiopathic PAH Activity at 5HT Transporter54-55 Associated? Odds Ratio Details Fenfluramines Substrate + 8 to 23 OR for >3-6 months use5359 Aminorex Substrate + ~98 OR based on population incidence in that era55-56 Methamphetamine Substrate + ~10 Only 1 study (K Chin)60 MDMA (ecstasy) Substrate -No dataAmphetamine Weak Substrate ? 1.4 (0.6-3.3) Studied diet-pill use only, p=NS59 Methylphenidate None -No dataCocaine* Reuptake inhib ? 2.8 (0.5-16) For “cocaine or IVDA”, p=NS, use was uncommon61 SSRIs – adults Reuptake inhib ? 0.1 (0.01-1.1)62, 0.4 (0.1-0.9) 63, 1.55 (1.1-2.1) 64 (details below**) Table: The association between diet-pills and IPAH (and possibly Meth and IPAH) is thought to likely relate to 5HT signaling. *Despite the low OR, in more recent years up to 80% of cocaine in the US is contaminated with, ironically, a drug that is metabolized to aminorex (the 1960’s diet-pill) – making it important to re-assess66. **While SSRIs are protective in animals, epidemiologic studies are mixed and unfortunately flawed and/or limited: too few patients exposed in one study62, improper controls in another63, and a third used administrative databases64 – problematic because of frequent miscoding65. II.B Innovation: Our proposal adds to the literature in several key areas. First, we propose that methamphetamine and MDMA are associated with idiopathic PAH. Our prior case-control study was conducted via chart review, while the proposed study will rely on in-person interviews. This will allow the inclusion of a larger number of medications and better information on route, duration and frequency of use. Second, we propose that only methamphetamine and MDMA are associated with idiopathic PAH, based on underlying mechanisms. If we identify an association between idiopathic PAH and both methamphetamine and MDMA and fail to find an association with the commonly used non-5HT transporter substrate drugs (amphetamine and methylphenidate), such a finding will add considerable support to the 5HT hypothesis in PH. Conversely, if we find that all evaluated stimulants are associated with IPAH, this would certainly require Research Strategy Page 88 Principal Investigator/Program Director (Last, first, middle): confirmation in a larger multicenter study, but would at least suggest a renewed look at adrenergic signaling in PAH. Finally, we will explore whether idiopathic PAH is associated or inversely associated with SSRIs and with 5HT receptor agonists such as migraine medications - though information for these two classes of medication will mainly be of use for planning future studies, based on our sample size estimates. II.C Approach: As s oc iations B etween S erotonin T rans porter S ubs trates and Idiopathic P AH Overview: The association between medication use and PH will be evaluated using a case-control study conducted by interview. Planned enrollment is 70 idiopathic PAH cases and 140 controls, made up of 70 “Other PH” patients and 70 normal controls. The controls will be matched as a group by age and gender. Subjects will undergo a 20 minute survey with questions on stimulants, diet-pills, antidepressants, antimigraine medications and others, as listed in table 7. Prior use of methamphetamine or MDMA, 5HT transporter substrates in common use today, will be the primary end-point. Table 7: Survey Medications Serotonin transporter substrates: Fenfluramine, dexfenfluramine, methamphetamine, MDMA Norepinephrine transporter substrates: Methamphetamine, MDMA, amphetamine, methylphenidate, others Serotonin transporter reuptake inhibitors: SSRIs (strong inhibitor), tricyclics and other antidepressants / also cocaine Serotonin receptor agonists: Ergotamine and triptan antimigraine medications Serotonin precursor: L-tryptophan Exploratory medications: Estrogen and progesterone “Control” medications* Benzodiazepines, narcotics (oral), marijuana Any prior use of the above types of medications will be recorded. This will be accomplished by asking about 60 specific medications, listed by name, and asking patients to name any other diet-pill, stimulant or antidepressant used. A reference list and picture chart with generic, brand and street names available will be used to help with recall. *In order reduce recall bias, patients will not be told the underlying hypothesis and suspected medications will be interspersed with medications that are not suspected. II.D Preliminary Studies: UCSD study60: In my first case-control study, I found that prior stimulant use was significantly more common among the 97 idiopathic PAH cases compared with the 243 other PH controls. After adjustment for age, patients with idiopathic PAH were approximately 9 times more likely to have a history of stimulant use than patients in the control group (p<0.001). This was driven mainly by methamphetamine use, and cocaine and amphetamine use alone were uncommon in idiopathic PAH. Methamphetamine was subsequently classified as “likely” associated with idiopathic PAH, based mainly on our report24,67. Recent studies in the neuropsychiatry literature provide a possible mechanism: methamphetamine has stronger 5HT transporter substrate activity than other stimulants, including methylphenidate (none) and amphetamine (weak)68. Based on the pattern we found, we now hypothesize that 5HT transporter substrate activity is the key factor in the association. UTSW study: Patients at UTSW with idiopathic PAH (N=48) also had higher rates of stimulant use, particularly methamphetamine use, compared with controls with other forms of pulmonary hypertension. Patients were included if they participated in a gene bank study which included chart review to determine any prior stimulant drug exposure. Limitations: use of chart review created several limitations including missing and incomplete data, lack of route of exposure, and lack of information on other medications – all of which can be better evaluated using the in-person survey that will be used in the proposed study. Table 8: Stimulant use (%) in Idiopathic PAH UCSD UTSW IPAH (N=97) Controls (N=243) Odds Ratio IPAH (N=48) Controls (N=89) Odds Ratio Any Meth* 23% 3% 10 (4.1-30) 19% 1% 14 (2.6-120) Meth alone 16% 2% 13% 0% Meth + cocaine 7% <1% 6% 1% Amphet. + cocaine 1% <1% 0% 0% Cocaine alone 1% 1% 2% 3% Amphetamine alone 3% 0% 8% 3% Any stimulant* 29% 4% 9.1 (4.2-23) 31% 8% 5 (1.8-17) Results: Any stimulant use and any meth use were higher in IPAH (idiopathic PAH) compared with controls (p<0.05*). Research Design and Methods: Study Subjects and Inclusion / Exclusion II.E Study subjects: Cases are patients with idiopathic PAH (N=70) recruited from the UTSW PH clinic. PHcontrols are patients with other forms of PH (N=70) as below. No-PH controls (N=70) are unaffected patients recruited from the UTSW Internal Medicine clinics. The population of patients with PH at UTSW includes more than 1000 established patients and another 250 new patients each year. This includes 120 patients with an Research Strategy Page 89 Principal Investigator/Program Director (Last, first, middle): idiopathic PAH diagnosis who are within 3 years of diagnosis and more than 600 patients with other forms of PH within 3 years of diagnosis. We estimate that 80% of those approached will agree to participate based on pilot survey results. Table 9: Inclusion Exclusion Criteria for Study Subjects in Case-Control Study Idiopathic PAH Cases: Inclusion: Age 18-70, idiopathic PAH as defined by the WHO consensus statement diagnosed within 3 years29, Complete work-up (VQ or CTA, echo, PFTs, cath mPAP >25 mmHg and wedge <15 mmHg). (Re: Age cutoff - drug use is less common above / below). Exclusion: All other forms of PH including familial PAH, connective tissue disease, congenital heart disease, left heart disease, lung disease and chronic PE. (2) HIV, HBV and HCV (Re: these infections are associated with both PAH and illicit drug use). PH Controls: Inclusion: Age 18-70, PH diagnosed within 3 years, Complete work-up (VQ or CTA, echo, PFTs, cath with mPAP >25 mmHg), PH subtypes of: connective tissue disease, congenital heart disease (non-ASD), left heart disease, lung disease and chronic PE. Exclusion: HIV, HBV and HCV and WHO group V conditions (miscellaneous and not well understood causes) and ASD-PAH No-PH Controls: Inclusion: Age 18-70, Seen in the UTSW Internal Medicine clinic. Exclusion: (1) Known PH. (2) PH risk factor: connective tissue disease, congenital heart disease, left heart disease (reduced LVEF or prior admission for CHF), lung disease (moderate or greater restrictive or obstructive lung disease or need for oxygen) and chronic PE. (3) Symptoms of PH (dyspnea, chest pain, ≥ moderate peripheral edema, syncope). (4) HIV, HBV and HCV. Inclusion / exclusion will be based on chart review; we will not actively screen because idiopathic PAH is rare (~20 per million) and usually highly symptomatic. Table: The PH classification consists of five major groups; idiopathic PAH (cases) are the subgroup of WHO group I (PAH) for which no other cause is identified. Other types of PH are the remaining WHO group I (PAH) subtypes (connective tissue disease, congenital heart disease and others), WHO group II (left heart), WHO group III (lung) and WHO group IV (chronic PE). These groups together are included as “Other PH” controls, except atrial septal defect (ASD) patients, a PAH subtype, are excluded because it is occasionally difficult to differentiate an ASD from a patient with idiopathic PAH and a patent foramen ovale. WHO group V (miscellaneous causes) is also excluded because this category is not well defined. Because correct classification is critically important, two independent pulmonary hypertension physicians will confirm all diagnoses prior to enrollment; if there is a disagreement the patient will be excluded. All groups: the survey will be in English, so English speakers only will be recruited. Rationale for control groups and study design: Case and control groups were selected based on the principles outlined in the classic 1992 series of articles by Wacholder et al. including use of techniques to minimize selection bias, differential errors in measurement (in cases vs. controls) and confounding. Wacholder suggests paying attention to the “study base”69-70, meaning the population from which the cases originated. When cases are identified in a referral center (as is typical with rare diseases), use of a disease control group is suggested because patients who travel to a referral center are likely different, on average, from patients who don’t. We therefore will use patients with other (non-idiopathic) forms of PH as the primary comparator; such an approach minimizes bias, particularly because type of PH is often unknown prior to referral. We will also include a second control group consisting of unaffected patients from the Internal Medicine clinic, which should strengthen the confidence in our results. Confounding will be addressed by adjusting risk estimates for prespecified confounders using a multivariate model and by group matching for age and gender. We will start by targeting the known gender and age distribution of our idiopathic PAH clinic patients, with age strata to include: 18-23, 24-29, 30-34, 35-39…65-70), and we will enroll cases and controls simultaneously with adjustment in the targeted strata if needed. Finally, recall bias will be reduced by improving recall overall through the use of a picture chart, by reducing group related differences by using a disease control group, by blinding patients and the interviewer to the study hypothesis, and by inserting dummy medications into the survey to reduce the likelihood of guessing the hypothesis. Survey: A 4 part survey will be administered in English at the time of routine office visit. The interviewer will obtain basic demographic information (gender, race, age, zip code of residence) and prior use of medications, as below. Over the counter stimulants (cold medications, mainly) are excluded because we found poor recall during pilot testing and because formulas have changed in recent years. A partial list of medications is listed in table 7. The survey will also include information on dose, date of last use, age of first use, and duration of use for prescription drugs, and date of last use, age at first use and total years of use. For illicit drugs, route of use (oral, nasal, smoked or intravenous) and frequency of use in days per week, days per month or total number of days in a 12 month period (in a typical year). This is asked this way because intermittent use is common. II.F Study Procedures: Eligible patients will be identified by review of records in our electronic medical record. Permission to approach patients will be obtained from their attending physician. Informed consent will be obtained from patients who agree to participate. The survey takes approximately 20 minutes based on pilot testing. Patient’s names will be recorded separately with a study ID number. A research coordinator will Research Strategy Page 90 Principal Investigator/Program Director (Last, first, middle): complete the survey by interview and using study forms. Survey development: Some questions are modeled after the addiction severity index (a public domain survey) and the NSDUH (US government) survey of illicit drugs. However, because we are interested in overall exposure rather than addiction for both prescription and recreational drugs, most of the questionnaire is unique. The survey has been piloted in 8 PAH patients and 5 no-PH controls. Changes so far include larger images on the picture card and elimination of over-the-counter medications due to poor recall. Once the survey modification is complete and the study begins, I will monitor survey administration by direct observation for 5% of interviews to reinforce standardization and consistency. Regulatory: IRB approval and an NIH certificate of confidentiality have been obtained. II.G Statistical Analysis Aim 2: To determine whether medications that increase serotonin transporter activity are associated with an increased risk of PAH by carrying out a case-control study Overview: 70 cases and 140 gender matched controls (70 no-PH and 70 other PH controls) will be enrolled, with age comparability ensured by frequency matching of the groups. The primary hypotheses is that increased prior methamphetamine and MDMA will be reported in patients with idiopathic PAH. 1. Demographics: Means and standard deviations, or when appropriate, medians and semi-interquartile ranges will be used to describe demographic and clinical characteristics. 2. Evaluation of the data: Stepwise multinomial logistic regression analyses will be performed using group (idiopathic PAH, PH control, No-PH control) as the dependent variable and with covariates of age, gender and race (black, white, other), alone and in combination, using p to enter of 0.1 and p to exit of 0.2. Three medications of interest will be included: MDMA, methamphetamine and combined methylphenidate and amphetamine, grouped because of similar mechanism, patterns of use and indication. p<0.05 will be considered significant. We will not adjust for multiple comparisons because of the small number of comparisons and because our overall hypothesis is focused on the two serotonin transporter substrates. 3. Anticipated control group use rates, effect size and power: Nationally, methamphetamine use rates range from 6-9%, MDMA use rates are approximately 5% and use of amphetamine or methylphenidate is 7% (individual use rates unavailable). Use of methamphetamine in North Texas specifically is estimated at 8%; use in Texas overall exceeds national averages71-73. We will have 80% power to detect an OR of 3.7 or greater for methamphetamine use alone, an OR of 4.95 for MDMA alone, and an OR of 4 or higher for amphetamine plus methylphenidate (alpha=0.05). We anticipate considerably higher ORs for methamphetamine (as high as an OR of 10-14) based on our two prior chart reviews. 4. Missing data: missing data for key variables will lead to exclusion of that subject. Exploratory evaluations: OR will also be determined for: (1) SSRIs, (2) Cocaine, (3) Antimigraine medications, and (4) Estrogens; these comparisons are exploratory and adjustment for multiple comparisons using standard techniques will be performed. Power overall was not determined, but is probably low for several medication of interest because: (1) Any association with SSRIs is probably no more than moderate, based on prior studies, (2) Antimigraine medication use is too infrequent. This data will thus be hypothesis generating and of use in determining appropriate sample sizes for future studies. Potential Problems: (1) Problems with enrollment: we have had high rates of enrollment into prior similar studies and >80% of patients approached during the pilot study testing have agreed, but if problems are seen we would extend enrollment by 1 year. (2) Underreporting of illicit medications: differential underreporting is discussed in the “recall bias” section above. As a general issue, we will reassure patients that their information will not be kept in their medical chart and their name will be kept separate from the survey. (3) Cautions due to sample size: a smaller but still elevated OR could be clinically important – and although this seems unlikely for the primary endpoint, the importance of this question as well as potentially whether any of the exploratory medications are associated will require evaluation in a larger study. We feel that the current study is the best combination of power and feasibility to test our primary hypothesis, but that a future multi-center study should be considered, once our analysis is complete. Cautions in interpretation: bias is always a concern in casecontrol studies. We will collect and review other demographic and medical information in order to look for other potential contributors to any observed associations and will obtain information as carefully as possible. Abbreviations CO: cardiac output / CI: cardiac index QIDS-SR: quick inventory of depressive symptomtology PA: pulmonary artery RA: right atrial; mPAP: mean pulmonary artery pressure 5HT: serotonin PAH*: pulmonary arterial hypertension SvO2: mixed venous oxygen saturation 5HIAA: 5-hydroxyindoleacetic acid PH*: pulmonary hypertension UCSD: University of California San Diego 6MWD: six minute walk distance PVR: pulmonary vascular resistance UTSW: UT Southwestern, Dallas Tx WHO: World Health Organization *PAH in humans refers exclusively to disease subtypes categorized as WHO group 1; PH is more general (all forms of pulmonary hypertension) Research Strategy Page 91 Principal Investigator/Program Director (Last, first, middle): Protection of human subjects Index to the Human Subjects Section: 1. Part 1: Clinical Trial A. Human subjects (characteristics and inclusion / exclusion) B. Sources of material C. Potential risks i. Medications ii. Catheterization iii. Other D. Adequacy of protections against risks E. Importance of knowledge to be gained / IND exemption F. Data and Safety Monitoring Plans G. ClinicalTrials.GOV Reporting 2. Part 2: Case-Control Study A. Human Subjects, Inclusion / Exclusion B. Sources of material C. Potential risks D. Adequacy of protection against risks Part 1 Fluoxetine Randomized Controlled Clinical Trial A. Human subjects involvement, characteristics, design Background: A randomized placebo controlled clinical trial will be completed where 34 patients are randomized to fluoxetine vs. placebo. Duration will be 24 weeks with a primary endpoint of change in PVR. Study population: Patients with idiopathic PAH, drug / toxin associated PAH or connective tissue disease PAH who are between 16 and 80 and who are receiving treatment with approved PAH therapies but remain symptomatic (WHO functional class II or III) with abnormal hemodynamics (PA mean >25, PVR >5 Wood units) will be enrolled. We anticipate 75% of enrolled patients will be functional class III. 1. Class I: no symptoms with ordinary activity 2. Class II: short of breath with ordinary activity 3. Class III short of breath with less than ordinary activity. 4. Class IV: short of breath at rest or with any physical activity. Patients will be recruited through the UTSW pulmonary hypertension clinic. All patients will be chronically ill; survival from diagnosis with PAH averages 5 years and patients with persistent class III symptoms after treatment have an average survival time of 2-3 years. Table 2: Inclusion / Exclusion Criteria Inclusion 1. WHO Group I PAH subtypes of IPAH, drugs / toxins, or connective tissue disease associated PAH, diagnosed by complete workup (VQ or CTA, echo, labs, PFTs, cath)* 2. Age 16-80 3. WHO Functional Class II or III (definition in “procedures” below) 4. Cath within 2 weeks of study entry with mPAP ≥ 25 mmHg, wedge ≤ 15 mmHg, and PVR ≥ 5 Wood units**. 5. Contraception use, (-) urine pregnancy test, not breast feeding (women of childbearing potential)† 6. One or more approved PAH therapies for ≥3 months, no change in dose for 1 month (endothelin-1 antagonist, phosphodiesterase5 inhibitor, prostacyclin / prostacyclin analog). Novel therapies in one of the three existing classes will also be acceptable as background therapy if approved during the course of the study; novel therapies in other classes will be excluded Exclusion 1. WHO Functional Class IV or listed for lung transplant (Reason: may be too ill / unstable) 2. Other cause for pulmonary hypertension: all other WHO group I diseases (including but not limited to congenital heart disease, liver disease, HIV), and WHO Groups II-V (i.e. left heart disease, lung disease etc)29. a. FEV1/FVC < 70% and FEV1 < 60% predicted, or TLC < 60% predicted unless no more than mild lung disease clinically and by computed tomography imaging b. High probability VQ or positive CTA c. Left ventricular ejection fraction <40% 3. Depression 4. Severe liver, renal or other medical or physical disease preventing completion of the study procedures 5. Use of antidepressants within 3 months Protection of Human Subjects Page 92 Principal Investigator/Program Director (Last, first, middle): *ACCP consensus conference definitions; these sub-types in general respond similarly to PAH therapies30.. **Higher PVR may increase chance of benefit in PAH in general, based on other studies. †Contraceptive pill, implant, vaginal ring, IUD, abstinent, or vasectomised partner. The entry criteria above were chosen because: 1. Patients with PAH should be on one or more approved PAH background therapies, because monotherapy has been shown to be beneficial (see below) 2. Class II – III patients are included because these patients are symptomatic and may benefit from additional therapy 3. Class IV patients are excluded due to high mortality rates and frequent need for transplant; they also make up <5% of our patient population so this should not significantly limit enrollment 4. The study population is limited to idiopathic PAH, drug / toxin PAH and connective tissue disease PAH because these patient populations have similar pathological changes within the lung vasculature and respond in a similar manner to currently approved PAH therapies; other forms of PAH are excluded because of greater heterogeneity and less clear benefit from other PAH therapies. 5. Pregnant women are excluded because patients with PAH should not become pregnant and because mortality is 30-40% during the pregnancy. Further, SSRIs may cause neonatal problems and are avoided during pregnancy except when benefits clearly outweigh risks. Children under 16 are excluded because of possibly greater risks with SSRIs and definitely greater risk with the study catheterizations, due to the need for sedation. Patients over 80 are excluded due to high rates of comorbidities as well as higher rates of misdiagnosis (meaning most pulmonary hypertension in this age group is either heart failure or lung disease, even when initial presentation suggests idiopathic PAH). PAH medications: All patients must be on therapy: There are three classes of approved medications. All enrolled patients must be on a stable dose of one of these medications. Combination therapy is also permitted. 1. Endothelin antagonists: bosentan or ambrisentan. 2. Phosphodiesterase 5 inhibitors: sildenafil or tadalafil. 3. Prostacyclins: epoprostenol or treprostinil or iloprost. Inhaled, subcutaneous or intravenous. Intravenous epoprostenol has shown a survival benefit in a randomized clinical trial77; the other medications have been shown to lead to improvement in exercise capacity and symptoms. Additionally, a survival benefit in general with use of approved PAH medications has been suggested by meta-analysis of all placebo controlled studies78. Combination therapy benefits may also exist: adding sildenafil to epoprostenol was beneficial in one study in terms of symptoms and exercise capacity. However, adding two oral therapies together is less clearly beneficial. Therefore combination therapy is allowed but not required. See also the American Heart Association guidelines for review79. If additional therapies are approved during the study our current plan is to allow oral prostacyclins, prostacyclin analogs, endothelin antagonists and phosphodiesterase 5 inhbitors as long as no suspected interaction with SSIRs, but to exclude medications in novel classes. However, if this becomes an issue with regards to enrollment / feasibility we will revisit this issue and would consider a protocol amendment. Alternatives to this study: since all potential study patients will be on one or more therapies prior to enrollment, alternatives to enrollment are adding additional approved therapies including in particular intravenous therapy, and transplant. All patients will be informed of these treatment options during the informed consent process, and these options will also be included in the formal informed consent documents. 1. Intravenous therapy: prostacyclins are felt to be the most potent PAH therapy, but side effects are common and include headache, nausea and diarrhea and complications such as line infections and sudden (potentially fatal) interruptions due to iv access issues. Intravenous therapy is discussed with all patients with persistent class III symptoms and with select class II patients who have other signs of worse prognosis. 2. Lung transplant: better survival can be achieved if medical therapy is used to postpone transplant as long as possible (including, if possible, indefinitely), as current survival with transplant averages only 5-6 years. Thus transplant is discussed in patients with right ventricular failure (RA pressure above 15 mmHg or cardiac index below 2.4 L/min/m2 + clinical symptoms of RV failure) and patients with other signs of severe disease (RV failure by other measures, such as cardiac MRI), but is put off as long as possible. Participation in the study does not preclude urgent lung transplant evaluation, if deterioration is seen. Protection of Human Subjects Page 93 Principal Investigator/Program Director (Last, first, middle): Progression during the study: patients who decline during the study and require additional therapy must exit the study; when possible an end of study visit including catheterization will be completed. Of note, this will be indicated in most patients with progression: transplant listing requires catheterization to achieve a higher listing via an “exception”, almost always needed in PAH, and catheterization is usually performed prior to initiation of intravenous therapy so as to establish a pre-prostacyclin baseline severity. Recruitment We plan enrollment of 34 patients. We follow more than 300 patients with idiopathic PAH or connective tissue disease PAH, and see an additional 50-60 new PAH patients per year (out of a total referral population of more than 300 new patients yearly). We therefore anticipate no difficulties in enrolling 9-12 patients per year for three years. Inclusion / exclusion criteria were also carefully selected based on prior trial experience. This includes (1) allowing background therapy (both because we feel it should now be standard of care, and for better recruitment), and (2) no upper limit on walk distance (meaning it won’t compete with most phase III studies as walk distance is often a primary endpoint and 450 meters is the usual cutoff in that setting). The use of catheterization as an endpoint may limit participation by a few patients. However, all patients in this study will be very familiar with catheterization and most will have undergone annual catheterizations for several years. We anticipate approaching up to 50 patients over 3 years to enroll in either the open label or clinical trial. We estimate 20% will decline participating due to either the catheterizations or the experimental nature, based on our experience with other trials. We also anticipate 10-15% of patients screened will be ineligible due to use of an SSRI. If recruitment falls below target, we will apply for modifications of the study protocol to include (in order) (1) Inclusion of patients with congenital heart disease or HIV, and (2) Keeping the study open an extra year. Nine patients enrolled in the prior reserpine-fluoxetine (later fluoxetine only) study over nine months, and therefore we feel our enrollment targets are realistic. Inclusion of special populations: children 16 years and older will be included. This is justified because PAH in older children is a progressive, fatal condition with similar pathophysiology and response to treatment as PAH in adults. Other treatment options are very limited, and even lung transplant is not an optimal solution: the supply of organs is limited, and survival of those who are transplanted averages only 5 years. Younger patients are excluded because of the need for general anesthesia (at our institution) for right heart catheterization, making the procedure riskier. More importantly, there is also concern that SSRIs could be harmful in young children, because SSRIs have been associated with neonatal pulmonary hypertension and because neonatal and early childhood PAH may pathophysiologically different from the disease in older children and adults. Study group assignment: study group assignment will be based on random number generation (in blocks of four) followed by the creation of numbered envelopes. Dose selection: The maximum approved dose of fluoxetine will be used in this study – we anticipate that this will be well tolerated in 50% of fluoxetine patients, that another 20% will tolerate 40 mg, 20% will tolerate 20 mg and 10% will drop out. These are very rough estimates based on tolerability of these medications in general and our finding that (1) One of 6 patients dropped out in the pilot study due to poor sleep. (2) Maximum tolerated dose was 20 mg in one patient, 40mg in two and 80 mg in two. We are hoping to increase tolerability by increasing the uptitration period from four weeks to twelve weeks. We are continuing to use the maximum approved dose as a target because we feel this will result in better reduction in serotonin signaling than using a lower dose and because higher doses were more effective in animal studies of fluoxetine. Additionally, down titration for side effects will be allowed as well. Research sites: research will be performed at St. Paul University Hospital and the attached St. Paul University Hospital clinics on the UT Southwestern Medical Center campus. Catheterizations will be performed in the St. Paul catheterization lab, where more than 400 right heart catheterizations are performed annually. Additionally, 16 normal controls will be recruited for the 24 hour 5HIAA tests and platelet tests: these will be age-matched (proportionally) and will be recruited through PH clinic from spouses and other non- related family members. These subjects will complete a brief questionnaire and a 24-hour urine collection and a blood draw. Protection of Human Subjects Page 94 Principal Investigator/Program Director (Last, first, middle): B. Sources of material Information will be extracted by a research coordinator from electronic medical records (medical history, physical exams, prior catheterization result, VQ scan, CT scans, echocardiogram and laboratory results (HIV, hepatitis, connective tissue disease serologies, routine chemistry and hematology and liver panels)). Research records will include patient initials and a study ID number; the key to this ID number will be maintained in a separate locked cabinet. Generated records will include study entry exam, test results (QIDSSR depression score, 6-minute walk distance, WHO functional class, catheterization results), medication dose and any side effects, any adverse events during the study. Data will also be entered into computerized research records that will consist of an excel spreadsheet with only unique study identifiers. Paper records will be stored within locked cabinets within a locked office. Computer records will be stored on password protected computers within locked offices. Study researchers, research coordinators and assistants who are listed on the IRB approval will have access to the records. The blood and urine specimens will be saved and stored with only study identifiers. C. 1. 2. 3. 4. Potential risks: summary Medications: suicidal ideation with fluoxetine (see details) Catheterization: pneumothorax, vascular injury, death, other Other risks: risks with walk distance test, psychological stress related to questionnaires Confidentiality (1) Medication risks Fluoxetine: Fluoxetine is FDA-approved for depression and is widely used today in the United States. Its safety in PAH is unknown, but two retrospective studies found risk ratios for mortality with SSRI use in idiopathic PAH of less than one, suggesting the potential for overall benefit (HR 0.35 (0.14-0.88)25 and 0.53 (0.07-3.9)26. No randomized controlled data is available. Side effects may include: Common (>10%): nausea, diarrhea, dry mouth, anxiety, somnolence. Less common but serious: chest pain, suicidal ideation and suicide attempts. We feel that it is appropriate to proceed to human studies: (1) Human studies (tissue and epidemiology studies) support possible beneficial effects. (2) Animal data supports a positive effect in PAH; (3) The tolerability of fluoxetine is likely to be better than several of the medications in current use in PAH (see alternative therapies above) Precautions to minimize medication risks: 1. Because there is some possibility of suicidal ideation with fluoxetine (reported when used for depression), all patients will be warned of the signs and symptoms of depression, and we will monitor for symptoms of depression during monthly visits. A baseline and follow-up QIDS-SR depression scale will be performed. Patients who develop symptoms of depression during the study will be referred for psychiatric evaluation. 2. Pregnancy and lactation: fluoxetine is pregnancy category C and possibly unsafe with lactation. A negative pregnancy test and effective contraception (see inclusion criteria) will be required. (2) Catheterization risks: Catheterizations are performed routinely in pulmonary hypertension for diagnosis and monitoring for disease progression. Patients will undergo baseline and 24-week catheterizations as a part of the study, though the baseline catheterization can be a routine annual catheterization as long as it was performed in the UTSW catheterization lab, all measurements required were obtained, and enrollment is within two weeks. The increased number of catheterizations adds a small amount of risk. This includes risk of pneumothorax, injury to vascular structures and death. Risk will be minimized by routine use of ultrasound during internal jugular access, and use of fluoroscopy and EKG monitoring during catheter placement. In experienced centers, the complication rate is low (<1%), and the serious complication rate is very low (<0.1%)70. Our own serious adverse event rate remains below these rates, likely due in part to the universal use of ultrasound (now standard) for more than 5 years resulting in a 0% pneumothorax rate over five years. (3) Other risks Risks related to the other study procedures (blood drawing, six minute walk testing) are low and will be minimized by following routine precautions for blood drawing, and by monitoring oxygen saturation, heart rate and patient well being during walk testing. Patients are asked to stop walking if they feel dizzy, develop severe Protection of Human Subjects Page 95 Principal Investigator/Program Director (Last, first, middle): dyspnea, or develop chest pain, or if they appear unsteady. They are monitored closely by a trained operator during the test. Stress / anxiety: Patients will be asked to complete a QIDS-SR depression scale. This may make some patients uncomfortable. If required, patients may decline to answer questions. Confidentiality: Patients protected health information will be collected during the study. This will be protected in locked cabinets within locked offices, and by use of password protected computers in locked offices. Decompensation related or unrelated to study medication: worsening pulmonary hypertension is always a possibility and a concern. Patients will be receiving closer than usual monitoring through more frequent visits and catheterizations. However, decompensation may occur. Any patient with clinical worsening who requires additional therapies will be withdrawn from the study, after undergoing an early “end of study” visit, when it is felt to be safe to do so and when they are willing. Additional therapy, as appropriate, will then be considered. D. Adequacy of Protections Against Risks Recruitment and Informed Consent Patients will be recruited through the pulmonary hypertension clinic. No paid advertising is planned – we maintain a good relationship with referring physicians in the community who are aware that “clinical trials” in general are available through our center. Once a potential patient is identified, I will review their chart for final determination of eligibility and if eligible, patients will be approached to enroll. Informed consent will be obtained by the enrolling physician (myself when possible; one sub-investigator will also participate). Patients are provided with a written copy of the informed consent, generally at a screening rather than enrollment visit. This allows them time to review the consent and ask any questions. After questions are answered, the consent and HIPAA documents will be signed and the patient will be given a copy of the consent to keep. The consent form includes detailed information about the nature of the research, the use of a placebo (for the clinical trial), the study procedures, the risks involved, and alternatives to participation. A study visit schedule is also included. For minors 16-17, patient assent plus parental consent (at least one parent) will be obtained. Because the children included are near the age of consent, these patients will be fully included in the discussion of risk vs. benefit, including review and an “assent” signature on the consent form and any answering any questions they may have. Minors over 18 are legally able to consent for themselves in our location, and thus parental involvement will not be required. Protections against risk 1. Medications: risk is reduced by limiting enrollment to class II/III patients, by excluding patients with hypotension or depression, and by close monitoring 2. Catheterization: risk is reduced by use of ultrasound, fluoroscopy, EKG monitoring and oxygen saturation monitoring. 3. Confidentiality: risk is reduced through the use of password protected computers, locked offices and cabinets, and avoiding patient names on research paperwork 4. Other unknown risks: close patient monitoring (visits monthly) will be performed in order to identify any other unexpected adverse events. E. Importance of knowledge to be gained, potential benefits PAH is a devastating, progressive condition. A better understanding of the role for serotonin may lead to improved treatments, and hopefully, improve survival beyond the current average of five years. Patients included in this study will continue to be monitored closely, and will continue to receive (at a minimum) the level of care they would have received outside the study. Investigational New Drug (IND) Exemption: The fluoxetine clinical trial should be IND exempt based on our discussions with our IRB and the FDA. For the pilot study (completed), our own IRB was uncertain as to whether the PAH patients were a subject population with “significantly increased risks” (criteria 3) and requested IND submission prior to approval; after submission the FDA classified it as IND exempt. We therefore anticipate similar classification with the slightly larger study in the same population. Specifically: IND exemption criteria: (1) it is not intended to be reported to FDA in support of a new indication for use or to support any other significant change in the labeling for the drug; (2) it is not intended to support a significant change Protection of Human Subjects As a small study looking at a surrogate endpoint (change in PVR), this will not support a label change or new indication. Same as response to (1) Page 96 Principal Investigator/Program Director (Last, first, middle): in the advertising for the product; (3) it does not involve a route of administration or dosage level, use in a subject population, or other factor that significantly increases the risks (or decreases the acceptability of the risks) associated with the use of the drug product; (4) it is conducted in compliance with the requirements for IRB review and informed consent [21 CFR parts 56 and 50, respectively]; (5) it is conducted in compliance with the requirements concerning the promotion and sale of drugs [21 CFR 312.7]; (6) it does not intend to invoke 21 CFR 50.24. The pilot study of fluoxetine and reserpine (see preliminary data section) met this criteria, per FDA response to our IND application. The fluoxetine study alone has a similar or lower risk (because only fluoxetine is used) and should therefore be similarly exempt. IRB review is being obtained for the clinical trial. Use of fluoxetine will be investigational; promotional claims of safety and effectiveness for PAH will not be made We will obtain informed consent F. Data and safety monitoring plans: Monitoring will include: 1. Principal investigator: As the principal investigator, I will have the primary responsibility for the safe conduct of the study. This will include careful conduct of the study, close monitoring for any adverse effects, and frequent follow-up. 2. Institutional review board (IRB): the UTSW IRB will monitor the study through review and approval of the protocoal prior to any patient enrollment, through routine review of any serious adverse events, through rereview every 6 months, and through general oversight of all research conducted at UT Southwestern. 3. Internal safety committee: A local safety monitoring committee is planned. This will include 3 physicians who are independent from the conduct of the study. A charter outlining responsibilities will be created. The chair of the committee will review all SAEs and these will be submitted to the chair at the same time they are submitted to the IRB. The full committee will meet every 6 months and review all SAEs along with a tabulated chart of all AEs every 6 months (tabulated and stratified by group assignment, with blinding maintained). Unblinded data will be provided to the committee if requested for safety reasons only, and if required, the study investigators will remain blinded. Adverse events will also be reported to the NIH and IRB, as required. G. ClinicalTrials.Gov Reporting We will register the randomized controlled clinical trial as required. Part 1 Case-Control Study A. Human subjects involvement and characteristics Study subjects: Cases are patients with idiopathic PAH (N=70) recruited from the UTSW PH clinic. Idiopathic PAH is a diagnosis of exclusion, requiring catheterization (PA mean >25 mmHg, wedge <15 mmHg) + absence of other associated diseases; a full work-up must have been completed, as per the 2009 classification scheme. Controls are patients with other forms of PH (N=70) as well as no-PH patients without pulmonary hypertension (N=70) recruited from the UTSW PH clinic and Internal Medicine clinics, respectively. Table 9: Inclusion Exclusion Criteria for Study Subjects in Case-Control Study Idiopathic PAH Cases: Inclusion: Age 18-70, IPAH as defined by the WHO consensus statement diagnosed within 3 years29, Complete work-up (VQ or CTA, echo, PFTs, cath mPAP >25 mmHg and wedge <15 mmHg). (Re: Age cutoff - drug use is less common above / below). Exclusion: All other forms of PH including familial connective tissue disease, congenital heart disease, left heart disease, lung disease and chronic PE. (2) HIV, HBV and HCV (Re: these infections are associated with both PAH and illicit drug use). PH Controls: Inclusion: Age 18-70, PH diagnosed within 3 years, Complete work-up (VQ or CTA, echo, PFTs, cath with mPAP >25 mmHg), PH subtypes of: connective tissue disease, congenital heart disease, left heart disease, lung disease and chronic PE. Exclusion: HIV, HBV and HCV No-PH Controls: Inclusion: Age 18-70, Seen in the UTSW Internal Medicine clinic. Exclusion: (1) Known PH. (2) PH risk factor: connective tissue disease, congenital heart disease, left heart disease (reduced LVEF or prior admission for CHF), lung disease (moderate or greater restrictive or obstructive lung disease or need for oxygen) and chronic PE. (3) Symptoms of PH (dyspnea, chest pain, ≥ moderate peripheral edema, syncope). (4) HIV, HBV and HCV. Inclusion / exclusion will be based on chart review; we will not actively screen with testing. This should not contaminate the control groups as IPAH is rare (~20 per million) and highly symptomatic. Table: Survey will be in English, so English speakers only will be recruited in all groups. Protection of Human Subjects Page 97 Principal Investigator/Program Director (Last, first, middle): Updated clinical classification of pulmonary hypertension 1 PAH 1.1 Idiopathic 1.2 Heritable 1.3 Drugs and toxins induced 1.4 Associated with (APAH) 1.4.1 Connective tissue disease 1.4.2 HIV 1.4.3 Portal Hypertension 1.4.4 Congenital Heart Disease 1.4.5 Schistosomiasis 1.4.6 Chronic haemolytic anemia 1.5 Persistent pulmonary hypertension of the newborn 1’ Pulmonary veno-occlusive disease and / or pulmonary capillary haemangiomatosis 2 Pulmonary hypertension due to left heart disease 2.1 Systolic dysfunction 2.2 Diastolic dysfunction 2.3 Valvular disease 3 Pulmonary hypertension due to lung disease and / or hypoxia 3.1 Chronic obstructive pulmonary disease 3.2 Interstitial lung disease 3.3 Other pulmonary diseases with mixed restrictive and obstructive pattern 3.4 Sleep disordered breathing 3.5 Alveolar hypoventilation disorders 3.6 Chronic exposure to high altitude 3.7 Developmental abnormalities 4 Chronic thromboembolic pulmonary hypertension 5 PH with unclear and / or multifactorial mechanisms (details omitted; includes sarcoidosis, vasculitis and others) Pulmonary Hypertension Classification: (from Guidelines published in 200927, 29). Idiopathic PAH patients are cases, other PH controls includes specifically the connective tissue disease and congenital heart disease subgroups from the group 1 along with all subsets of groups 2, 3 and 4. Conditions which are associated with drug use (hepatitis, HIV) are excluded, and patients with congenital heart disease related to an atrial septal defect are excluded because this can be difficult to differentiate from idiopathic PAH with a patent foramen ovale, in some cases. No-PH controls are patients from the Internal Medicine Clinic without known PH, PH symptoms or PH risk factors including such as connective tissue disease, heart failure, lung disease and prior PE. B. Sources of material Information will be obtained by interview and by extraction from electronic medical records (medical history, physical exams, prior catheterization result, VQ scan, CT scans, echocardiogram and laboratory results (HIV, hepatitis, connective tissue disease serologies, routine chemistry and hematology and liver panels)). Research records will be generated documenting (1) Demographics, and (2) Medication and drug use questions. Because of the sensitive nature, patient names will not be recorded on the questionnaire. Instead, a study number will be created. The key to the questionnaires will be maintained in a separate, double locked cabinet until the analysis is complete, at which point it will be destroyed. Study researchers, research coordinators and assistants who are listed on the IRB approval will have access to the records; access to the key will be limited to the principal investigator and one coordinator. C. Potential risks The main risks of this study relate to confidentiality (and legal ramifications of any breach, in cases of illicit behavior), and psychological stress related to the survey. D. Adequacy of Protections Against Risks The risk of inadvertent disclosure will be minimized through the use of locked cabinets within a locked office, computer records without names or other specific identifiers (only study numbers) and stored on password protected computers within locked offices, and by limiting the number of individuals with access to the records. Additionally, legal risk has been minimized by obtaining a certificate of confidentiality from the NIH; this minimizes the ability of courts to subpoena study documents. Psychological stress will be minimized by letting patients know that this is voluntary and confidential (i.e. won’t be a part of any medical records), and that they should not feel they are under any pressure to participate. E. Importance of knowledge to be gained, potential benefits Idiopathic PAH is a devastating, progressive condition. A better understanding of the role for medications in the development of the disease may help identify new targets to block, and allow the avoidance of risky medications, when possible. Protection of Human Subjects Page 98 Principal Investigator/Program Director (Last, first, middle): Inclusion of Women and Minorities (Both Clinical Trial and Case-Control Study) Minorities: We will actively recruit patients of all races and ethnicities into the clinical trial and the case-control study. In prior studies, our demographics have been representative of North Texas in general (see chart below showing our largest study). We anticipate a similar breakdown in both the clinical trial and in the case-control study. Spanish language – clinical trial: We are particularly well suited to enroll Spanish speaking patients (approximately 4% of all of our patients are Spanish speaking only or prefer Spanish) as our coordinators and one physician are fluent. We routinely use a Spanish language consent for our clinical trials, and will do so in the fluoxetine study. A QIDS-SR depression scale and SF-36 in Spanish are also available. Non-English speaking patients with other primary languages and an inability to converse in English are rare and will not be offered enrollment; we do offer enrollment in later phase (phase 3 and 4 studies) because the potential for benefit is generally somewhat greater and the risk is usually better established, but in this very early phase study we feel safety monitoring could be compromised. Case-control study: we will only enroll English speaking patients as cases and controls, due to the survey-based methodology. This study will be too small to test and validate the survey in other languages. However, we plan to eventually conduct a larger multi-center case-control study and will anticipate including Spanish speaking participants in this future study. Table: reported race in Texas and in a UTSW IPAH study, and anticipated racial break-down based on a prior moderately large (for PAH) study. White Black Native American Asian or pacific islander (separate sub-group data unavailable) Texas, 2005 84%* 12% 1% 3.6% UTSW IPAH patients participating 84%* 11% 0% 5% in a genetic study in 2009 (N=71) Ethnicity: 34% of Texas respondents report Hispanic ancestry (most with White race); of the UTSW IPAH patients this was 18%. This lower value is likely due to the predominantly North Texas referral area where the number of Hispanic individuals is lower than in South Texas. Gender: Both genders will be included, with an anticipated 3:1 female to male ratio based on the demographics of PAH (it is more common in women). Outreach programs: We do not recruit directly to patients because PAH is rare. However, we do accept indigent patients and patients with only Medicaid, and we do periodically inform referring physicians of ongoing studies. We also have a particularly close affiliation with Dallas Counties’ Parkland Memorial Hospital: Parkland is affiliated with UT Southwestern, our pulmonary fellows and many of our UTSW faculty rotate on-service there, and we are frequently referred patients. Parkland is a 968 bed county hospital that provides both primary care and tertiaryreferral level care for Dallas County residents. Parkland’s demographics include 47% Hispanic and 31% African American patients, and so this outreach helps to broaden the demographics of our referral pool. Women &Minorities Page 99 Principal Investigator/Program Director (Last, first, middle): *Note two separate tables, one for each study: Inclusion Enrollment Report This report format should NOT be used for data collection from study participants. Role for Serotonin in IPAH – Fluoxetine Sub-Project Study Title: Total Enrollment: 34 Protocol Number: Grant Number: PART A. TOTAL ENROLLMENT REPORT: Number of Subjects Enrolled to Date (Cumulative) by Ethnicity and Race Sex/Gender Unknown or Ethnic Category Females Males Not Reported Hispanic or Latino Total 5 1 0 6 21 7 0 28 0 0 0 0 26 8 0 34 American Indian/Alaska Native 0 0 0 0 Asian 2 0 0 2 Native Hawaiian or Other Pacific Islander 0 0 0 0 Black or African American 3 1 0 4 21 7 0 28 More Than One Race 0 0 0 0 Unknown or Not Reported 0 0 0 0 26 8 0 34 Not Hispanic or Latino Unknown (individuals not reporting ethnicity) Ethnic Category: Total of All Subjects* ** * Racial Categories White Racial Categories: Total of All Subjects* * PART B. HISPANIC ENROLLMENT REPORT: Number of Hispanics or Latinos Enrolled to Date (Cumulative) Racial Categories Females Unknown or Not Reported Males Total American Indian or Alaska Native 0 0 0 0 Asian 0 0 0 0 Native Hawaiian or Other Pacific Islander 0 0 0 0 Black or African American 0 0 0 0 White 5 1 0 6 More Than One Race 0 0 0 0 Unknown or Not Reported 0 0 0 0 Racial Categories: Total of Hispanics or Latinos** 5 1 0 6 * These totals must agree. ** These totals must agree. Planned Enrollment Table Page 100 ** Principal Investigator/Program Director (Last, first, middle): Inclusion Enrollment Report This report format should NOT be used for data collection from study participants. Role for Serotonin in IPAH – Case-Control Sub-Project Study Title: Total Enrollment: 210 Protocol Number: Grant Number: PART A. TOTAL ENROLLMENT REPORT: Number of Subjects Enrolled to Date (Cumulative) by Ethnicity and Race Sex/Gender Unknown or Ethnic Category Females Males Not Reported Hispanic or Latino Total 28 10 0 38 129 43 0 172 0 0 0 0 157 53 0 210 American Indian/Alaska Native 0 0 0 0 Asian 8 3 0 11 Native Hawaiian or Other Pacific Islander 2 1 0 3 19 6 0 25 128 43 0 171 More Than One Race 0 0 0 0 Unknown or Not Reported 0 0 0 0 157 53 0 210 Not Hispanic or Latino Unknown (individuals not reporting ethnicity) Ethnic Category: Total of All Subjects* ** * Racial Categories Black or African American White Racial Categories: Total of All Subjects* * PART B. HISPANIC ENROLLMENT REPORT: Number of Hispanics or Latinos Enrolled to Date (Cumulative) Racial Categories Females Unknown or Not Reported Males Total American Indian or Alaska Native 0 0 0 0 Asian 0 0 0 0 Native Hawaiian or Other Pacific Islander 0 0 0 0 Black or African American 1 0 0 1 28 9 0 37 More Than One Race 0 0 0 0 Unknown or Not Reported 0 0 0 0 29 9 0 38 White Racial Categories: Total of Hispanics or Latinos** * These totals must agree. ** These totals must agree. Planned Enrollment Table Page 101 ** Principal Investigator/Program Director (Last, first, middle): Inclusion of Children: Clinical Trials Children 16 and older will be included in the fluoxetine study (clinical trial). Younger children are excluded because the disease process is probably different in infants and may be different in young children99, and because cardiac catheterization is a higher risk procedure for children as it requires much higher levels of sedation or general anesthesia. Fluoxetine may also be associated with increased risk of neonatal pulmonary hypertension. This could be because SSRIs reduce growth but worsen vasoconstriction, as pulmonary hypertension in infants seems to be a more vasoconstrictive process. Although school age children probably have physiology more closely related to the adult process, this study will limit enrollment to the population we feel are most likely to benefit and have lower risk of harm from study medications and study procedures. If fluoxetine is found to be beneficial in older children and adults, then additional study in younger children is anticipated. Inclusion of Children: Case-Control Study In the case-control study, children under 18 are excluded because the average age of first use of methamphetamine ranges from age 19-22, and because exposure may not lead to disease for several years. We therefore have limited the patient population to those 18 and over. We also chose to limit the study to individuals under 70 because both PAH and drug use in elderly patients are uncommon100. Children Page 102 Principal Investigator/Program Director (Last, first, middle): Resource Sharing Plan: The data generated by these two studies will be published as expeditiously as possible, in order to facilitate sharing of any information gained. Resource Sharing Plan Page 103 SUMMARY STATEMENT ( Privileged Communication ) PROGRAM CONTACT: Sandra Colombini-Hatch 301-435-0222 hatchs@nhlbi.nih.gov Release Date: Application Number: 08/08/2012 1 K23 HL105784-01A1 Principal Investigator Applicant Organization: UNIVERSITY OF TEXAS SW MED CTR/DALLAS Review Group: ZHL1 CSR-X (O1) National Heart, Lung, and Blood Institute Special Emphasis Panel K23, K24, K25 Research Career Development Awards Meeting Date: 06/28/2012 Council: OCT 2012 Requested Start: 09/01/2012 RFA/PA: PCC: PA11-194 LLLJAN Project Title: The Serotonin Transporter in Pulmonary Arterial Hypertension SRG Action: Human Subjects: Animal Subjects: Gender: Minority: Children: Project Year 1 2 3 4 ___________ TOTAL Impact/Priority Score: 13 30-Human subjects involved - Certified, no SRG concerns 10-No live vertebrate animals involved for competing appl. 1A-Both genders, scientifically acceptable 1A-Minorities and non-minorities, scientifically acceptable 1A-Both Children and Adults, scientifically acceptable Clinical Research - not NIH-defined Phase III Trial Direct Costs Requested 119,175 119,175 119,175 119,175 _______________ 476,700 Estimated Total Cost 128,589 128,589 128,589 128,589 _______________ 514,356 ADMINISTRATIVE BUDGET NOTE: The budget shown is the requested budget and has not been adjusted to reflect any recommendations made by reviewers. If an award is planned, the costs will be calculated by Institute grants management staff based on the recommendations outlined below in the COMMITTEE BUDGET RECOMMENDATIONS section. 1 K23 HL105784-01A1 2 ZHL1 CSR-X (O1) 1K23HL105784-01A1 Notice: All Career Development (K) applications received after January 25, 2010 will be required to use the restructured applications forms and conform to the new page limits. See NOT-OD-10-002 for further clarification. (http://grants.nih.gov/grants/guide/notice-files/NOT-OD-10-002.html) NHLBI “K” series resubmission/amended application due date is Mar. 12, July 12, or Nov. 12. In addition, a Letter of Intent (LOI) is requested from applicants who are planning to submit a resubmission application. The LOI should be submitted by February 12, June 12, or October 12 (one month in advance of the submission date). Submit LOI to nhlbichiefreviewbranch@nhlbi.nih.gov Note: New NIH Policy on Resubmission (amended) Applications Please see the following link for clarification: http://grants.nih.gov/grants/guide/notice-files/NOTOD-09-003.html Please be advised that NIH has adopted a new policy for accepting Post-Submission Application Materials. Please see the following link and related notices for a description of acceptable and unacceptable post-submission application materials: http://grants.nih.gov/grants/guide/noticefiles/NOT-OD-10-091.html CRITIQUES The comments in the CRITIQUE section were prepared by the reviewers assigned to this application and are provided without significant modification or editing by staff. They are included to indicate the range of comments made during the discussion and may not reflect the final outcome. The RESUME AND SUMMARY OF DISCUSSION section summarizes the final opinion of the committee after discussion and is the basis for the assigned priority score. RESUME AND SUMMARY OF DISCUSSION: This is a resubmission of a K23 application from . The reviewers agreed that was highly responsive to the previous review criticisms and has produced more focused and improved career development and research plans. The candidate has been very productive since the original submission, publishing six manuscripts and obtaining intramural funding to support her preliminary studies that were the basis for this proposal. The mentoring team is still considered outstanding, but the reviewers were disappointed that the mentors did not update their biosketches. Overall, the application was rated in the exceptional range – exceptionally strong with essentially no weaknesses. DESCRIPTION (provided by applicant): Candidate: MD, is an Assistant Professor in Pulmonary and Critical Care at the University of Texas Southwestern Medical Center in Dallas, TX. She seeks support for career development to emerge as an independent investigator in the area of pulmonary arterial hypertension. Environment: Mentorship will be provided by Dr. Milton Packer, an expert in cardiovascular clinical trials, with additional mentorship from Drs. Lewis Rubin, an expert in pulmonary hypertension, and Lance Terada, an expert on cell signaling. Research and Career Development: proposes two studies that will focus on the role for the serotonin transporter in pulmonary arterial hypertension (PAH). These two studies will test the hypothesis that the serotonin transporter is a key contributor to the development and persistence of elevated pulmonary pressures in patients with pulmonary arterial hypertension (PAH). The first study will be a randomized double-blind placebo controlled trial evaluating whether blockade of the serotonin transporter with fluoxetine, a selective serotonin reuptake inhibitor, will reduce pulmonary vascular resistance in patients with PAH. In her preliminary work involving open label use of fluoxetine, found that three months treatment led to improvement in cardiac index (p<0.05) and pulmonary vascular resistance (p=NS) compared with baseline. In the proposed clinical trial, 34 patients will be randomized to placebo or fluoxetine for six months. The primary endpoint will be change in pulmonary vascular resistance, and secondary endpoints will include other hemodynamic changes, exercise capacity, quality of life and 1 K23 HL105784-01A1 3 ZHL1 CSR-X (O1) safety. second study will evaluate whether use of stimulants and other medications that act as serotonin transporter substrates is associated with idiopathic PAH. This will be studied using a casecontrol design with 70 cases and 140 controls, and medication use will be determined by in-person survey. has previously shown that idiopathic PAH may be associated with use of methamphetamine, a stimulant with serotonin transporter substrate activity. proposes to supplement this practical training in clinical research with formal coursework in statistics, pharmacology and pulmonary vascular biology. This work addresses several National Heart Lung and Blood Institute strategic objectives, including (1) Improving the understanding of the molecular and physiological basis of health and disease, and (2) Improving the understanding of the clinical mechanisms of disease and thereby enabling better prevention, diagnosis, and treatment. CRITIQUE 1: Candidate: 2 Career Development Plan/Career Goals /Plan to Provide Mentoring: 1 Research Plan: 2 Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s): 2 Environment and Institutional Commitment to the Candidate: 1 Overall Impact: This is a very responsive, revised application from an Assistant Professor at the University of Texas, Southwestern (UTSW) who is currently the Director of the UTSW pulmonary hypertension program at this institution. She has been very focused on translational work in pulmonary hypertension (PAH). She has published eight peer reviewed papers focusing on PAH, although only two in the past two years. She has extensive previous experience in conducting industry-sponsored trials but the rationale for additional training and mentoring is now well described. She has already completed a master’s program. In response to previous reviews the career development plan (CDP) has been appropriately revised to maximize additional training. Additional, compelling preliminary data are presented which has led to significant changes in the research plan; this is now considered to be much improved. The overall hypothesis regarding the role of serotonin (5HT) transporter remains quite fascinating. The revised research plan includes a randomized controlled trial (RCT) of fluoxetine in idiopathic PAH. The second specific aim remains a case-control study examining the role of methamphetamine and MDMA exposure with PAH. The mentoring team remains outstanding, as does institutional support. 1. Candidate: Strengths The application is strengthened by an outstanding candidate. She is well trained having completed pulmonary training at UCSD, an outstanding center for translational pulmonary vascular research. She successfully competed for an F32 NRSA at UCSD but turned down the award as she was moving to UTSW to focus on clinical pulmonary hypertension work. After initial work as a clinician at an affiliated institution she returned to UTSW in 2007 as an Assistant Professor. While there she successfully competed for a KL2 award and completed a Master of Science in Clinical Sciences in 2011. She has published 8 peer reviewed manuscripts and eight chapters/review articles. Six of these have been since returning to UTSW and are clearly focused in clinical pulmonary vascular disease. She has been an investigator in numerous industry-sponsored trials giving her a good sense of the complexity of clinical trials in human PH. She received highly competitive internal CTSA funding to complete a pilot study, which had been the first aim of the previous K23 submission. 1 K23 HL105784-01A1 4 ZHL1 CSR-X (O1) Weaknesses Limited publication in the past year. 2. Career Development Plan/Career Goals & Objectives/Plan to Provide Mentoring: Strengths She enumerates a clear long-term goal of developing an independent career in PH research. In response to the previous review the CDP has been altered and is considered quite responsive to the previous reviews. She has clearly defined what additional training is required. In this capacity she has chosen advanced biostatistical didactic training in the CTSA partner institution, the University of North Texas Health Science Center School of Public Health. This institution provides epidemiology and population focused statistical training. The time commitment for this course work is well defined and seems quite feasible. In direct response to previous reviewer recommendations she will take additional coursework in pulmonary vascular biology and pharmacology. Finally, focused training in grant writing and scientific presentation will be pursued. Weaknesses None 3. Research Plan: Strengths The research plan has been significantly altered since the prior submission. The overall hypothesis remains quite interesting in suggesting that 5HT transporter is a key modulator of PH. The epidemiological preliminary data are quite compelling. The original submission included a pilot study of reserpine and fluoxetine. Limited preliminary data at that time was felt to be a moderate limitation. In the current application describes the results of that pilot study – reserpine was abandoned due to poor tolerance and fluoxetine seemed to provide functional improvement, albeit with difficulties in drug tolerance. Given completion of the pilot study the revised application now presents two complementary and better defined and supported specific aims. The first specific aim flows from the pilot study and will be a randomized controlled trial of fluoxetine in Class I PH. The dosage schedule has been altered based on the pilot study with monthly increases in dose. In response to previous reviews, drug exposure has been expanded to six months with additional clinical follow-up to 12 months. The primary endpoint remains PVR but additional clinical (PRO) endpoints have been added. Focused substudies have been added to address 5HIAA levels and platelet function. These seem quite appropriate and provide additional potential mechanistic insight. The second specific aim remains similar to that originally proposed but is supported by additional chart review data from UTSW (results quite similar to previous ones from UCSD). A case-control study comparing idiopathic PAH (n=70), non-idiopathic PAH (n=70), and healthy controls (n=70) will be recruited. In person interviews will be completed to assess exposure to methamphetamine and MDMA. Other relevant drug exposures will be sought as well. Weaknesses The exact mechanism for determination of dose increase is not clearly specified. There are minor inconsistencies in study conduct of the RCT including; a) duration of drug exposure; b) how LVEDP will be measured in some; and c) the timing of 6MWT and right heart catheterization. All of these are considered relatively minor limitations. Should the primary statistical analyses adjust for baseline PVR? The presence of a local DSMB is quite appropriate although its structure is likely not optimal. Given the pilot study results, meetings more frequently than six months seems appropriate. 1 K23 HL105784-01A1 5 ZHL1 CSR-X (O1) Given anticipated age differences between idiopathic PAH and non-idiopathic PAH, it is not clear how straightforward age matching will prove to be. 4. Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s): Strengths The mentoring team remains outstanding. The primary mentor remains Milton Packer who is an international authority in clinical trials in heart failure (and pulmonary vasculopathy). He was the PI of the UTSW CTSA and runs the KL2 program currently. He is the Chair for the Department of Clinical Sciences at UTSW. He clearly specifies that will be his only primary mentee. His letter of support is exemplary. Dr. Lewis Rubin of UCSD, her previous mentor, remains a secondary mentor. The modality for his continued input is convincing. Dr. Lance Terada, her division chief at UTSW, provides valuable mechanistic insight and career advice. Three advisors with well-defined, complementary roles are described. These individuals will form an advisory committee with clearly specified roles and defined goals. Weaknesses Biosketches do not appear to have been updated. 5. Environment and Institutional Commitment to the Candidat: Strengths The institutional commitment remains outstanding. Dr. Johnson provides assurance of clear support for protected time. Drs. Johnson and Packer assure financial support for non-study related expenses. Additional clinical staff hired for the pulmonary hypertension program will provide administrative support. Weaknesses None Data and Safety Monitoring Plan (Applicable for Clinical Trials Only): Acceptable o With the caveat that more frequent review of AEs and SAEs is suggested. Resource Sharing Plans: Acceptable Acceptable but superficial. Recommended budget modifications or possible overlap identified: Pending resolution of the previous institution K support - it seems like she may have received one year of support. CRITIQUE 2: Candidate: 2 Career Development Plan/Career Goals /Plan to Provide Mentoring: 1 Research Plan: 1 Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s): 1 Environment and Institutional Commitment to the Candidate: 1 Overall Impact: 1 K23 HL105784-01A1 6 ZHL1 CSR-X (O1) The revised application is significantly responsive to the Summary Statement critiques, particularly in the presentation of preliminary data on efficacy and safety of the proposed intervention and revision of the Research Plan to include a placebo-controlled, randomized, clinical trial. The candidate is outstanding, and it is noted that she obtained a CTSC pilot grant and performed pilot studies that significantly modified her research proposal in an informed and insightful manner. The candidate is clearly motivated to pursue a patient-oriented research career, and she is clearly capable of doing so. 1. Candidate: Strengths is an outstanding candidate, with a good publication record and clear motivation to pursue a patient-oriented research career. The fact that she obtained a coveted CTSC pilot grant and performed a pilot study in the time since the original application is impressive on many levels, including that it is a reflection of motivation and ability to successfully apply for funding and achieve her goals. Weaknesses Few recent publications. 2. Career Development Plan/Career Goals & Objectives/Plan to Provide Mentoring: Strengths Excellent career development plan, including statistical training. Weaknesses None 3. Research Plan: Strengths Outstanding research plan including a placebo-controlled, randomized trial. Performance of pilot study and detailed and intelligent discussion of it, including of modifications made to the study design based on the results is a real strength of the proposal. Weaknesses None 4. Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s): Strengths Outstanding mentors Primary mentor will meet with the candidate weekly and states that he is the primary mentor to only one primary mentee at this time, ensuring adequate time for the mentee. A particularly detailed and informative letter from the mentor was very helpful and is appreciated. Weaknesses None 5. Environment and Institutional Commitment to the Candidate: Strengths Outstanding environment, including a strong track record of studies in this area and a CTSC program. Weaknesses None Resubmission: Very responsive to previous review Recommended budget modifications or possible overlap identified: 1 K23 HL105784-01A1 7 ZHL1 CSR-X (O1) Please clarify KL2 support from 2008-2011: In other support -- 3 years. In Biosketch -- 1 year. CRITIQUE 3: Candidate: 2 Career Development Plan/Career Goals /Plan to Provide Mentoring: 2 Research Plan: 2 Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s): 4 Environment and Institutional Commitment to the Candidate: 2 Overall Impact: This is a revised K23 application from at the UNIVERSITY OF TEXAS SW MED CTR/DALLAS seeking 4 years of mentored training to become an independent clinical trialist and investigator in pulmonary arterial hypertension (PAH). The original application was favorably reviewed two years ago; however, the two most concerning aspects were a lack of preliminary data demonstrating feasibility to study the combination of fluoxetine and reserpine in treatment of PAH and the problem of Aims 2 and 3 being dependent on the positive outcome of this treatment combination (original Aim 1). The safety concerns of using SSRI therapy and potential risk of suicide ideation was also raised. Additional suggestions were made to improve the study design and data collection in the research strategy. There was also some confusion as to why the PI needed additional protected time for mentored training beyond the KL2 experience and the acquisition of a master’s degree in Clinical Investigation—the justification for (e.g., survival analysis) and type of (e.g., lack of training in vascular biology) additional didactic training was incomplete. Minor concerns about the mentoring team oversight were also raised such as why the PI has not published with her mentor, Dr. Packer, and the remote nature of the mentoring relationship with Dr. Rubin at UCSD. The original Aim 1 was deleted from the revision, as this aim was to collect the preliminary data using the combined treatment of fluoxetine and reserpine. Pilot studies carried out by the PI showed that risks to patients outweighed the potential benefits of reserpine therapy. Data collected using fluoxetine alone suggested a potential therapeutic benefit on cardiac index and pulmonary vascular resistance (PVR) in patients with PAH. These data demonstrate feasibility for the randomized, double blind placebo controlled clinical trial of 34 patients with PAH to determine whether fluoxetine lowers PVR and other important outcome measures including QOL. Aim 2 will be a case controlled study to determine whether medications that increase 5HT transporter activity [methamphetamine and 3,4-methylenedioxymeth-amphetamine (MDMA)] are associated with an increased risk of PAH. Significant effort was put into the revised proposal to address details of inclusion/exclusion criteria, control populations, safety monitoring, data analysis, as well as limitations of the study. The PI has gained significant experience already as a subinvestigator on many clinical trials, which is reflected in the design of the proposed clinical studies. The original review considered the candidate, the mentoring team and the research environment and institutional commitment to the PI to be outstanding. These criteria remain outstanding and the PI and primary mentor responded to the minor concerns adequately. There is a remaining confusion as to how many years the PI was supported as a KL2 scholar (1 yr or 3 years?) as discrepant values were found in the text of the proposal. In summary, this project addresses an important unmet clinical need to find better therapies to treat PAH and provides the training opportunities for the PI to become an independent principal investigator of clinical trials and translational research in PAH. 1. Candidate: Strengths PI remains outstanding and has additional first or senior authored peer-reviewed publications Letters of support unanimously strong 2. Career Development Plan/Career Goals & Objectives/Plan to Provide Mentoring: 1 K23 HL105784-01A1 8 ZHL1 CSR-X (O1) Strengths Clarification of the need for additional training in advanced epidemiology, statistics, survey design and pharmacology was provided and training in vascular biology was added. 3. Research Plan: Strengths The research plan was significantly revised to provide preliminary data to support feasibility of the study and to simplify the RCT design to test only one therapeutic intervention, namely fluoxetine. Additional outcomes measures were included to make the clinical trial more robust 4. Mentor(s), Co-Mentor(s), Consultant(s), Collaborator(s): Strengths The mentoring team remains outstanding experientially and each member is clearly committed to the PI’s training and transition to independence. Weaknesses Although Dr. Packer indicated that he purposefully does not included himself as a co-author on many of his trainees’ papers, it would seem important to publish a couple of papers with each trainee to demonstrate that a true mentoring relationship was established (and the trainee may be proud to publish with his/her mentor!) and that the mentor is supportive of the research plan and the veracity of the data outcomes. Most of the mentors’ grants have ended and the publications listed in Biosketches are not very up to date (most recent: Packer, 2006; Rubin, 2008; Terada, 2009 and PLoS One, in press). Essentially, it looks like none of the mentors provided updated Biosketches since the original K23 grant submission in 2010 (eek). 5. Environment and Institutional Commitment to the Candidate: Strengths Institutional support remains strong with the promise of additional funds ($30K) to support the research and that her position is not contingent on the K23. Should the K23 be awarded, her time will be protected at 75%-effort to carry out the studies proposed. Protections for Human Subjects: Acceptable Risks and Adequate Protections very detailed Data and Safety Monitoring Plan (Applicable for Clinical Trials Only): Acceptable o described nicely Resubmission: Concerns of prior review were addressed appropriately. Additional Comments to Applicant (Optional): Presenting text in tabular form (Tables 4 and 5) is a bit dodgy as it looks like an attempt to circumvent page limitations. Also, using bullet points for describing significance is unusual grant style. Together, these two styles interspersed with "normal" text makes for a choppy grant reading experience. (End of Reviewers’ Comments) 1 K23 HL105784-01A1 9 ZHL1 CSR-X (O1) THE FOLLOWING RESUME SECTIONS WERE PREPARED BY THE SCIENTIFIC REVIEW ADMINISTRATOR TO SUMMARIZE THE OUTCOME OF DISCUSSIONS OF THE REVIEW COMMITTEE ON THE FOLLOWING ISSUES: PROTECTION OF HUMAN SUBJECTS (Resume): ACCEPTABLE; the committee recommends that the DSMB meet more frequently than every six months. INCLUSION OF WOMEN PLAN (Resume): ACCEPTABLE INCLUSION OF MINORITIES PLAN (Resume): ACCEPTABLE INCLUSION OF CHILDREN PLAN (Resume): ACCEPTABLE BIOHAZARD COMMENT: NONE TRAINING IN THE RESPONSIBLE CONDUCT OF RESEARCH: ACCEPTABLE COMMITTEE BUDGET RECOMMENDATIONS: RECOMMENDED AS REQUESTED ADMINISTRATIVE NOTE: Clarify the duration of candidate’s K12 funding. NIH has modified its policy regarding the receipt of resubmissions (amended applications). See Guide Notice NOT-OD-10-080 at http://grants.nih.gov/grants/guide/notice-files/NOT-OD10-080.html. The impact/priority score is calculated after discussion of an application by averaging the overall scores (1-9) given by all voting reviewers on the committee and multiplying by 10. The criterion scores are submitted prior to the meeting by the individual reviewers assigned to an application, and are not discussed specifically at the review meeting or calculated into the overall impact score. For details on the review process, see http://grants.nih.gov/grants/peer_review_process.htm#scoring.