Diatomic molecules
advertisement
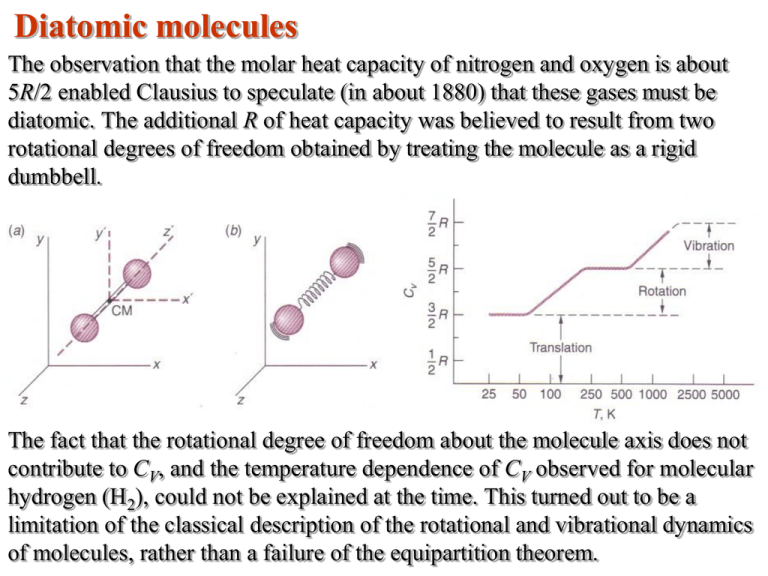
Diatomic molecules The observation that the molar heat capacity of nitrogen and oxygen is about 5R/2 enabled Clausius to speculate (in about 1880) that these gases must be diatomic. The additional R of heat capacity was believed to result from two rotational degrees of freedom obtained by treating the molecule as a rigid dumbbell. The fact that the rotational degree of freedom about the molecule axis does not contribute to CV, and the temperature dependence of CV observed for molecular hydrogen (H2), could not be explained at the time. This turned out to be a limitation of the classical description of the rotational and vibrational dynamics of molecules, rather than a failure of the equipartition theorem. CV for solids The equipartition theorem is also useful for understanding the heat capacity of solids. Dulong and Petit pointed out that the molar heat capacity for most solids is very nearly equal to 3R. The six contributions of ½R come from the three kinetic energy terms (½mv2, etc.) and the three potential energy terms (½kx2, etc.). R = 2 kcal/kmol.K At high temperatures, all solids obey the Dulong and Petit law. For temperatures below come critical value (called the Debye temperature, which is a characteristic of the material), the value of CV drops significantly. Again, this turns out to be a limitation of the classical theory. Also puzzling is the fact that free electrons in metals do not appear to contribute to CV. Equipartition of energy 1 2 1 2 1 2 1 2 kT 1 mv mv x mv y mv z 3 3 mvi2 2 2 2 2 2 2 • Here, vi represents one component of velocity. • On average, one expects each component to contribute equally to the kinetic energy, since the distribution function must be isotropic. • Therefore, one can see that each degree of freedom, f, contributes 1/2kT to the average kinetic energy of a molecule.. • Thus, the total internal energy of a gas is given by, kT f U N fN nRT 2 2 and U f u RT . n 2 Gas pressure and the ideal gas law Kinetic theory provides a natural interpretation of the absolute temperature of a dilute gas. Namely, the temperature is proportional to the mean kinetic energy ( ) of the gas molecules. • The mean kinetic energy is independent of pressure, volume, and the molecular species, i.e. it is the same for all molecules. 1 2 1 2 2 2 pV Nmv N mv N NkT 3 3 2 3 2 p u (u is an energy density) 3