PHZ6426: Fall 2013 Problem set # 4: Crystal Structure
advertisement
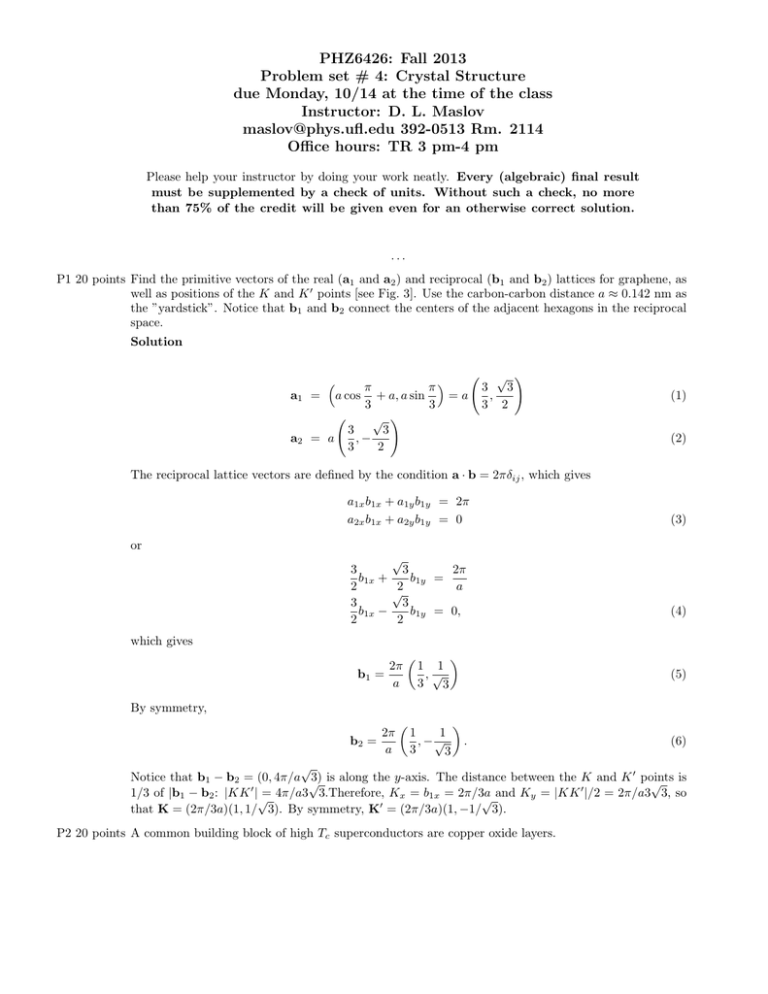
PHZ6426: Fall 2013 Problem set # 4: Crystal Structure due Monday, 10/14 at the time of the class Instructor: D. L. Maslov maslov@phys.ufl.edu 392-0513 Rm. 2114 Office hours: TR 3 pm-4 pm Please help your instructor by doing your work neatly. Every (algebraic) final result must be supplemented by a check of units. Without such a check, no more than 75% of the credit will be given even for an otherwise correct solution. ... P1 20 points Find the primitive vectors of the real (a1 and a2 ) and reciprocal (b1 and b2 ) lattices for graphene, as well as positions of the K and K 0 points [see Fig. 3]. Use the carbon-carbon distance a ≈ 0.142 nm as the ”yardstick”. Notice that b1 and b2 connect the centers of the adjacent hexagons in the reciprocal space. Solution a1 a2 π π = a cos + a, a sin =a 3 3 √ ! 3 3 ,− = a 3 2 √ ! 3 3 , 3 2 (1) (2) The reciprocal lattice vectors are defined by the condition a · b = 2πδij , which gives a1x b1x + a1y b1y = 2π a2x b1x + a2y b1y = 0 (3) or √ 2π 3 3 b1x + b1y = 2 2 a √ 3 3 b1x − b1y = 0, 2 2 (4) which gives 2π b1 = a 1 1 ,√ 3 3 (5) By symmetry, 2π b2 = a 1 1 , −√ 3 3 . (6) √ Notice that b1 − b2 = (0, 4π/a √ 3) is along the y-axis. The distance between the K and K 0 points √ is 0 0 1/3 of |b1 − b2 : |KK |√= 4π/a3 3.Therefore, Kx = b1x = 2π/3a and K = |KK |/2 = 2π/a3 3, so y √ 0 that K = (2π/3a)(1, 1/ 3). By symmetry, K = (2π/3a)(1, −1/ 3). P2 20 points A common building block of high Tc superconductors are copper oxide layers. A possible choice of the primitive cell. The cell contains 1 Cu atom and 2 O atoms =O =Cu FIG. 1: Unit cells for a flat (2D) CuO2 plane and for a real (3D) CuO2 sheet. • Sketch the Bravais lattice, identify the basis, and define the primitive unit cell for a 2D CuO2 plane, as shown in Fig. 4. • In a parent compound of high Tc superconductors ( LaCuO4 ), the oxygen atoms are displaced above and below the plane in an alternating fashion, as shown in Fig. 5. Identify the primitive unit cell for this case. Solution See Figures 1 and 2. P3 20 points p • Show that the c/a ratio for an ideal hexagonal close-packed (hcp) lattice is 8/3 ≈ 1.63. Solution Since the spheres centered at atoms in the basal plane touch each other, a = 2R, where R is the radius of the sphere. A sphere centered at point A must touch the one centered at point O, therefore, |OA| = 2R. If√A0 is the projection of A onto the basal plane, the distancep|OA0 | is equal to 0 0 2 0 2 (a/2)/ cos(30◦ )=a/√ 3. From √ the rectangular triangle OAA , c/2 = |AA |/2 = |OA| − |OA | . Therefore, c/a = 2 2/3 = 8/3. • Sodium transforms from the body-centered cubic (bcc) to hcp phase at T = 23 K. Assuming that the number density remains fixed and also that the c/a ratio remains ideal, find the hcp lattice spacing a given that the lattice spacing in the bcc phase is a0 = 4.23Å. =O above the plane =O below the plane =Cu FIG. 2: Unit cells for a real (3D) CuO2 sheet. Solution A bcc lattice can be viewed as a simple cubic lattice with 2 atoms per unit cell. √ Therefore, 0 3 the number density in the bcc phase n = 2/(a ) . The hcp unit cell of volume ( 3/2)a2√ c also √ 2 bcc √ contains 2 atoms, thus nhcp = 4/ 3a c. For an ideal hcp lattice, c = 8a/3 and nhcp = 4/ 8a3 . Equating nbcc and ntcp , we obtain a = a0 /21/6 = 3.77Å. P4 20 points Find the volumes of the primitive unit cells of the bcc and fcc cubic lattices. Solution For a bcc lattice, the primitive lattice vectors can be chosen as a (ŷ + ẑ − x̂) 2 a = (x̂ + ẑ − ŷ) 2 a = (x̂ + ŷ − ẑ) 2 a1 = a2 a3 (7) The volume of the primitive cell V = |a1 × (a2 × a2 )|. Simple vector calculus gives a1 × a2 = (a2 /2)(ŷ + ẑ), and V = a3 /2. (8) fcc lattice: a1 = a a a (ŷ + ẑ)a2 = (x̂ + ẑ)a3 = (x̂ + ŷ) 2 2 2 (9) V = a3 /4. P5 20 points Consider an ”imperfect crystal”, where the nth atom (for all n) is displaced from its ideal position, Rn , by a random vector Sn . We are interested in a diffraction peak around the reciprocal lattice vector G. Assume that the displacements at all sites are small: |Sn | a, where a is the lattice spacing. Find the intensity of the diffraction peak. Hint: Assume that all the three components of S are distributed according to the Gaussian law with 1 exp(−Si2 /2σ 2 ), where i = x, y, z. zero average P (Si ) = √2πσ 2 Solution The intensity of the scattered wave is proportional to the mod squared of the structure factor averaged over random displacements of atoms X I ∝ h| exp(iG · Rn ) exp(iG · Sn )|2 i n = X hexp(iGSn ) exp(iGSn0 )i, (10) n,n0 where Sm is the projection of Sm onto G. Assuming that displacements of different atoms are independent and obey Gaussian distributions with dispersions S0 , we obtain Z ∞ 1 hexp(iGSn )i = √ dSn exp(−S 2 /2S02 + iGSn ) 2πS0 −∞ Z 1 1 dSn exp − 2 Sn2 − 2iS02 GSn =√ 2S0 2πS0 Z 2 1 1 =√ dSn exp − 2 Sn − iS02 G + S04 G2 2S0 2πS0 Z z2 1 dz exp − 2 exp(−S02 G2 /2) =√ 2S0 2πS0 = exp(−S02 G2 /2). (11) Therefore, the intensity of the Bragg peak in the direction of G is attenuated by the factor exp(−G2 S02 ). This factor is known as the “Debye-Waller factor”. b1 a1 a2 K K' b2 FIG. 3: Graphene lattice in real (left) and reciprocal (right) spaces. =O =Cu FIG. 4: Flat (2D) CuO2 plane. =O above the plane =O below the plane =Cu FIG. 5: 2D projection of a real (3D) CuO2 sheet.





